HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2024-2025
Date & Time: 20th February 2025, 11:00 am
Duration: 3h
Advertisements
General instructions:
The question paper is divided into four sections:
- Section A: Q. No. 1 contains Ten multiple-choice type of questions carrying One mark each. Only the first attempt will be considered for evaluation. Q. No. 2 contains Eight very short answer type of questions carrying One mark each.
- Section B: Q. No. 3 to Q. No. 14 contain twelve short answer type - I of questions carrying two marks each. (Attempt any Eight).
- Section C: Q. No. 15 to Q. No. 26 contains Twelve short answer type - II of questions carrying Three marks each. (Attempt any Eight).
- Section D: Q. No. 27 to Q. No. 31 contain Five long answer type of questions carrying Four marks each. (Attempt any Three).
- Use of the log table is allowed. Use of calculator is not allowed.
- Figures to the right indicate full marks.
- Given data:
- R = 8.314 J/K/mol
- Atomic mass Na = 23
- Kf for water = 1.86 K kg mol-1
- 1F = 96500C
- NA = 6.022 × 1023
Schottky defect is NOT observed in ______.
NaCl
KCl
AgBr
NiO
Chapter:
The freezing point of 0.1 m aqueous solution of urea, if Kf for water is 1.86 K kg mol-1 is ______.
1.86 °C
−1.86 °C
0.186 °C
−0.186 °C
Chapter:
Ozone layer is depleted by ______.
NO
NO2
NO3
N2O5
Chapter: [0.07] Elements of Groups 16, 17 and 18
When an excess of AgNO3 is added to the complex, one mole of AgCl is precipitated. The formula of the complex is ______.
[CoCl2(NH3)4]Cl
[CoCl(NH3)4]Cl2
[CoCl3(NH3)3]
[Co(NH3)6]Cl3
[CoCl(NH3)5]Cl2
Chapter: [0.09] Coordination Compounds
The value of Δng for the oxidation of 4 moles of sulphur dioxide to sulphur trioxide is ______.
−2
2
−4
4
Chapter:
One dimensional nanostructure amongst the following is:
Nanoparticles
Nanotubes
Nanofilms
Nanorods
Chapter:
Which formula co-relates degree of dissociation and concentration of electrolyte?
`c = sqrt ((K_a)/α)`
`α = sqrt ((K_a)/c)`
`c = sqrt( K_a α)`
`c = sqrt (α/K _a)`
Chapter:
The highest acidic compound among the following is:
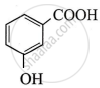
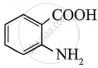
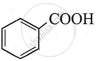
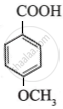
Chapter:
The formula used to calculate the molar conductivity of an electrolyte is ______.
`∧ = (1000c)/k`
`c = (1000∧)/k`
`∧ = (1000k)/c`
`k = 1000/(∧c)`
Chapter:
Which of the following is a secondary amine?
Cyclohexylamine
Isopropylamine
Diphenylamine
N, N-Dimethylaniline
Chapter:
Write the structural formula of N, N-dimethylethanamine.
Chapter:
Write the reagents used for the reduction of the carbonyl group in the Clemmensen reduction.
Chapter:
The rate law equation for \[\ce{A -> Product}\], is rate = k[A]x. What is the effect of an increase in concentration of ‘A’ on the rate of reaction if x < 0?
Chapter:
What is the molality of an aqueous solution of KBr having freezing point −3.72°C (Kf for water is 1.86 K kg mol−1)?
Chapter:
Write balanced chemical equation for the following:
When excess of ammonia is treated with chlorine.
Chapter:
Write the number of donor atoms present in EDTA during the formation of the complex.
Chapter:
Write the names of the metal elements in the brass alloy.
Chapter:
Derive an expression for the relation between half-life and rate constant for first-order reaction.
Chapter: [0.06] Chemical Kinetics
Define osmotic pressure.
Chapter: [0.02] Solutions [0.02] Solutions and Colligative Properties
Distinguish between lanthanoid and actinoids.
Chapter: [0.08] Transition and Inner Transition Elements [8.02] F-block Elements
Write anomalous behaviour of oxygen with respect to atomicity.
Chapter:
Write anomalous behaviour of oxygen with respect to the oxidation state.
Chapter:
Write anomalous behaviour of oxygen with respect to magnetic property.
Chapter:
Write anomalous behaviour of oxygen with respect to the nature of hydrides.
Chapter:
What is the action of liquid bromine in acetic acid on anisole?
Chapter:
What is the action of soda-lime on sodium acetate?
Chapter:
Calculate the work done in kJ in a reaction if the volume of the reactant decreases from 8 dm3 to 4 dm3 against 43 bar pressure.
[1 dm3 bar = 100 J]
Chapter:
Explain ionization isomers with suitable example in complexes.
Chapter:
Advertisements
Explain the preparation of glucose from sucrose.
Chapter: [0.14] Biomolecules
How many coulombs of electricity is required to produce 1g of sodium metal by reduction of sodium ion?
Chapter:
Write the structural formula and IUPAC name of the alcohol having molecular formula C4H10O which does not undergo oxidation under normal condition.
Chapter:
Identify ‘A’ and ‘B’ in the following reaction and rewrite the complete reaction:
\[\ce{CH3 - CH = CH2 ->[HBr][Peroxide] A ->[alcoholic KCN][KBr] B}\]
Chapter:
Write the reaction for the preparation of acetaldehyde by Rosenmund reaction.
Chapter:
Chapter:
Write the general electronic configuration of 3d series.
Chapter: [0.08] Transition and Inner Transition Elements
Draw the structure of the following:
H2SO4
Chapter: [7.02] Group 16 Elements
Write the molecular and structural formulae of thiosulfuric acid.
Chapter: [7.02] Group 16 Elements
Define conjugate acid-base pair.
Chapter: [0.03] Ionic Equilibria
The hydroxyl ion concentration in an aqueous solution of NaOH is 2 × 10−4 mol dm−3. Calculate PH of the solution.
Chapter:
Explain any two applications of nanomaterials.
Chapter: [0.16] Green Chemistry and Nanochemistry
Write the name and formula of the reagent used to convert alkylhalide to nitroalkane.
Chapter:
Write the reactions for the action of the following reagent on phenol:
Nitrating mixture
Chapter:
Write the reaction for the action of the following reagent on phenol:
Zinc dust
Chapter:
What is the action of phosphorous pentacholoride on ethyl methyl ether?
Chapter:
Explain the formation of [CO(NH3)6]3+ complex ions with respect to the type of hybridisation.
Chapter:
Explain the formation of [CO(NH3)6]3+ complex ions with respect to magnetic property.
Chapter:
Calculate spin only magnetic moment of M2+ ion.
[atomic number of M = 26]
Chapter:
Write the condensed electronic configuration of Gadolinium [Z = 64].
Chapter:
Advertisements
Name different zones in the blast furnace. Write the reactions taking place in them.
Chapter: [0.08] Transition and Inner Transition Elements
Write the chemical equation involved in the carbylamine reaction for ethylamine.
Chapter:
Write the chemical equation involved in the following reaction:
Hoffmann-bromamide degradation reaction
Chapter: [0.13] Amines [13.01] Amines
Explain Cannizzaro's reaction with the help of benzaldehyde.
Chapter:
Write the reaction for the conversion of cyclohexene to adipic acid.
Chapter:
Define “zero order reaction”.
Chapter: [0.05] Chemical Kinetics
A reaction takes place in two steps:
- \[\ce{NO_{(g)} + Cl2_{(g)} -> NOCl2_{(g)}}\]
- \[\ce{NOCl2_{(g)} + NO_{(g)} -> 2NOCl_{(g)}}\]
- Write the overall reaction.
- Identify the reaction intermediate.
- What is the molecularity of each step?
Chapter: [0.06] Chemical Kinetics
ΔH for the formation of ethane gas is −84.4 kJ at 300 K. Calculate ΔU for the reaction.
Chapter:
Mention the types of polymers formed on the basis of intermolecular forces.
Chapter:
Write any two uses of low density polyethylene.
Chapter:
An element with molar mass 27 g/mol forms a cubic unit cell with edge length of 405 pm. If the density of the element is 2.7 g/cm3, what is the nature of the cubic unit cell?
Chapter: [0.01] Solid State
Derive the equation of Raoult’s law for binary solution containing non-volatile solute.
Chapter:
State whether entropy change is positive or negative in the following example:
Melting of ice
Chapter:
State whether entropy change is positive or negative in the following example:
Vaporisation of a liquid
Chapter:
Explain the common ion effect with an example.
Chapter: [0.03] Ionic Equilibria
Draw a neat labelled diagram of a lead accumulator.
Chapter: [0.05] Electrochemistry
Write the overall reactions taking place at the cathode in a lead accumulator cell during discharging of the cell.
Chapter:
Write the overall reactions taking place at the anode in a lead accumulator cell during discharging of the cell.
Chapter:
Define unit cell.
Chapter: [0.01] Solid State [0.01] Solid State
Which colour is shown by NaCl crystal due to formation of F-centre?
Chapter:
Why does fluorine show anomalous behaviour in ‘17 group’ elements?
Chapter:
What is the action of the following reagent on bromomethane?
bromobenzene
Chapter:
What is the action of the following reagents on bromomethane?
Mercurous fluoride
Chapter:
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2024 - 2025
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2025 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
