Advertisements
Advertisements
Question
Describe the manufacture of H2SO4 by contact process?
Solution 1
Sulphuric acid is manufactured by the contact process. It involves the following steps:
Step (i):
Sulphur or sulphide ores are burnt in air to form SO2.
Step (ii):
By a reaction with oxygen, SO2 is converted into SO3 in the presence of V2O5 as a catalyst.
2SO2(g) + O2(g) 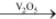 2SO3(g)
2SO3(g)
Step (iii):
SO3 produced is absorbed on H2SO4 to give H2S2O7 (oleum).
`SO_3+H_2SO_4 -> H_2S_2O_7`
This oleum is then diluted to obtain H2SO4 of the desired concentration.
In practice, the plant is operated at 2 bar (pressure) and 720 K (temperature). The sulphuric acid thus obtained is 96-98% pure
Solution 2
Preparation of sulphuric acid:By Contact Process: Burning of sulphur or sulphide ores in presence of oxygen to produce SO2. Catalytic oxidation of SO2 with O2to give SO3 in the presence of V2O5.
2SO2(g) + O2(g) 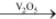 2SO3(g)
2SO3(g)
Then SO3 made to react with sulphuric acid of suitable normality to obtain a thick oily liquid called oleum.
`SO_3(g) + H_2SO_4(l) -> H_2S_2O_7(l)`
Then oleum is diluted to obtain sulphuric acid of desired concentration
`H_2S_2O_7(l) + H_2O(l) -> 2H_2SO_4(l)`
The sulphuric acid obtained by contact process is 96-98% pure
APPEARS IN
RELATED QUESTIONS
What is molecular formula of oleum?
(a) H2SO3
(b) H2SO4
(c) H2S2O7
(d) H2S2O8
What is the action of concentrated sulphuric acid on phosphorous pentachloride
What is the action of concentrated sulphuric acid on copper
When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was evolved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
1) Identify (A) and (B).
2) Write the structures of (A) and (B).
3) Why does gas (A) change to solid on cooling?
Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber process. Draw the structure of phosphorous pentachloride
Write a balanced chemical equation for the following reaction:
Sulphuric acid is treated with phosphorous.
Write the formula for pentaamminechlorocobalt (III) sulphate.
Write the reaction of conc. \[\ce{H2SO4}\] with sugar.
Identify the INCORRECT statement about H2SO4.
Gas 'X' is prepared by treating sodium sulfite with dilute sulfuric acid. When gas 'X' is oxidised by dioxygen in presence of vanadium (V) oxide, gas 'Y' is formed. Identify X and Y.
The molecular formula of oleum is ____________.
The sulfuric acid obtained by contact process is ____________ % pure.
Which among the following catalysts is used in manufacture of sulphuric acid by contact process?
Which of the following statements are correct?
(i) S – S bond is present in \[\ce{H2S2O6}\].
(ii) In peroxosulphuric acid \[\ce{(H2SO5)}\] sulphur is in +6 oxidation state.
(iii) Iron powder along with \[\ce{Al2O3}\] and \[\ce{K2O}\] is used as a catalyst in the preparation of \[\ce{NH3}\] by Haber’s process.
(iv) Change in enthalpy is positive for the preparation of \[\ce{SO3}\] by catalytic oxidation of \[\ce{SO2}\].
The number of dative bonds in sulphric acid molecule is ______.
What is the catalyst used for the oxidation of SO2 to SO3 in the lead chamber process for the manufacture of sulphuric acid?
