Advertisements
Advertisements
प्रश्न
Describe the manufacture of H2SO4 by contact process?
उत्तर १
Sulphuric acid is manufactured by the contact process. It involves the following steps:
Step (i):
Sulphur or sulphide ores are burnt in air to form SO2.
Step (ii):
By a reaction with oxygen, SO2 is converted into SO3 in the presence of V2O5 as a catalyst.
2SO2(g) + O2(g) 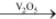 2SO3(g)
2SO3(g)
Step (iii):
SO3 produced is absorbed on H2SO4 to give H2S2O7 (oleum).
`SO_3+H_2SO_4 -> H_2S_2O_7`
This oleum is then diluted to obtain H2SO4 of the desired concentration.
In practice, the plant is operated at 2 bar (pressure) and 720 K (temperature). The sulphuric acid thus obtained is 96-98% pure
उत्तर २
Preparation of sulphuric acid:By Contact Process: Burning of sulphur or sulphide ores in presence of oxygen to produce SO2. Catalytic oxidation of SO2 with O2to give SO3 in the presence of V2O5.
2SO2(g) + O2(g) 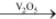 2SO3(g)
2SO3(g)
Then SO3 made to react with sulphuric acid of suitable normality to obtain a thick oily liquid called oleum.
`SO_3(g) + H_2SO_4(l) -> H_2S_2O_7(l)`
Then oleum is diluted to obtain sulphuric acid of desired concentration
`H_2S_2O_7(l) + H_2O(l) -> 2H_2SO_4(l)`
The sulphuric acid obtained by contact process is 96-98% pure
APPEARS IN
संबंधित प्रश्न
Write the conditions to maximize the yield of H2SO4 by contact process.
Complete the following equations: CaF2+H2SO4 →
Complete the following equations: C + conc. H2SO4 →
Mention three areas in which H2SO4 plays an important role.
Why is `K_(a_2) "<<" K_(a_1)` for `H_2SO_4` in water?
What is the action of concentrated sulphuric acid on phosphorous pentachloride
What is the action of concentrated sulphuric acid on copper
When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was evolved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
1) Identify (A) and (B).
2) Write the structures of (A) and (B).
3) Why does gas (A) change to solid on cooling?
Give balanced chemical equations for Sulphuric acid is treated with hydrogen sulphide
Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber process. Draw the structure of phosphorous pentachloride
Write a balanced chemical equation for the following reaction:
Sulphuric acid is treated with phosphorous.
Write the formula for pentaamminechlorocobalt (III) sulphate.
Identify the products formed when conc. sulfuric acid reacts with calcium fluoride.
Gas 'X' is prepared by treating sodium sulfite with dilute sulfuric acid. When gas 'X' is oxidised by dioxygen in presence of vanadium (V) oxide, gas 'Y' is formed. Identify X and Y.
The molecular formula of oleum is ____________.
The sulfuric acid obtained by contact process is ____________ % pure.
What is the ratio of volumes of gases involved in the preparation of sulphur trioxide from sulphur dioxide and dioxygen respectively under similar conditions of temperature and pressure?
Hot conc. \[\ce{H2SO4}\] acts as the moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following element is oxidised by conc. \[\ce{H2SO4}\] into two gaseous products?
