Advertisements
Advertisements
Question
In reference to Freundlich adsorption isotherm, write the expression for adsorption of gases on solids in the form of an equation.
Solution
Freundlich adsorption isotherm for adsorption of gases on solids:
xm=Kp1nIt can also be written aslogxm=log K + 1nlog p">
\[\frac{x}{m} = K p^\frac{1}{n} \]
\[\text{It can also be written as}\]
\[\log\frac{x}{m} = \log K + \frac{1}{n}\log p\]
where x is the mass of the adsorbate, m is the mass of the absorbent and p is the pressure of the gas and n is a constant which is greater than 1.
APPEARS IN
RELATED QUESTIONS
What is an adsorption isotherm?
Describe Freundlich adsorption isotherm.
Freundlich's equation for adsorption of gas on solid is represented as ____________.
Which of the following curves is in accordance with Freundlich adsorption isotherm?
Adsorption of gases on the solid surface is generally exothermic because.
In the freundilch adsorption isotherm, the value of x/m is 0.4 under a pressure of 0.2 at m calculate the value of the interact
In Freundlich adsorption isotherm, slope of AB line is:
The mass of gas adsorbed, x per unit mass of adsorbed, m was measured at various x pressures p. A graph between `log "x"/"m"` and log p gives a straight line with slope equal to 2 and the intercept equal to 0.4771. The value of `"x"/"m"` at a pressure of 4 atm is ______.
[Given log 3 = 0.4771]
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The x plot of log `x/m` versus log p is shown in the given graph. `x/m` is proportional to ______.
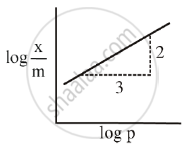
For Freundlich adsorption isotherm, a plot of log (x/m) (Y-axis) and log p (x-axis) gives a straight line. The intercept and slope for the line is 0.4771 and 2, respectively. The mass of gas, adsorbed per gram of adsorbent if the initial pressure is 0.04 atm, is ______ × 10-4 g. (log 3 = 0.4771)
