Advertisements
Advertisements
Question
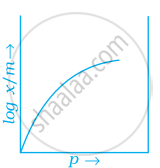
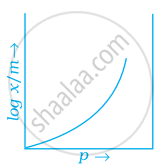
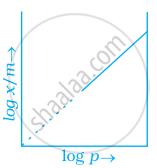
Which of the following curves is in accordance with Freundlich adsorption isotherm?
Options
Solution
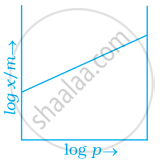
Explanation:
Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
The adsorption varies directly. Where
APPEARS IN
RELATED QUESTIONS
What do you understand by activation of adsorbent? How is it achieved?
Answer in one sentence:
Write an equation for Freundlich adsorption isotherm.
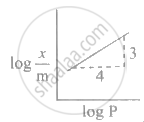
Explain graphically Freundlich adsorption isotherm.
The slope and intercept for plot of
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The plot of log
Freundlich's equation for adsorption of gas on solid is represented as ____________.
Freundlich adsorption isotherm is given by the expression
(i) When
(ii) When
(iii) When n = 0,
(iv) When n = 0, plot of
Which of the following statement is INCORRECT regarding the adsorption of a gas on the surface of the solid?
The mass of gas adsorbed, x per unit mass of adsorbed, m was measured at various x pressures p. A graph between
[Given log 3 = 0.4771]
For Freundlich adsorption isotherm, a plot of log (x/m) (Y-axis) and log p (x-axis) gives a straight line. The intercept and slope for the line is 0.4771 and 2, respectively. The mass of gas, adsorbed per gram of adsorbent if the initial pressure is 0.04 atm, is ______ × 10-4 g. (log 3 = 0.4771)