Advertisements
Advertisements
Question
What do you understand by activation of adsorbent? How is it achieved?
Solution 1
By activating an adsorbent, we tend to increase the adsorbing power of the adsorbent. Some ways to activate an adsorbent are:
(i) By increasing the surface area of the adsorbent. This can be done by breaking it into smaller pieces or powdering it.
(ii) Some specific treatments can also lead to the activation of the adsorbent. For example, wood charcoal is activated by heating it between 650 K and 1330 K in vacuum or air. It expels all the gases absorbed or adsorbed and thus, creates a space for adsorption of gases.
Solution 2
Activation of an adsorbent means increasing it’s adsorbing power by increasing the surface area of the adsorbent by making it’s surface rough, by removing already adsorbed gases from it and by subdividing the adsorbent into smaller pieces or grains.
APPEARS IN
RELATED QUESTIONS
What is an adsorption isotherm?
Describe Freundlich adsorption isotherm.
Answer in one sentence:
Write an equation for Freundlich adsorption isotherm.
Explain graphically Freundlich adsorption isotherm.
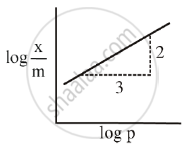
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The plot of log `"x"/"m"`· versus log P is shown in the given m graph. `"x"/"m"` is proportional to
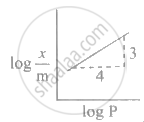
Freundlich's equation for adsorption of gas on solid is represented as ____________.
In Freundlich's adsorption isotherm, when log `("x"/"m")` is plotted against log P, slope of the graph is ____________.
Which of the following curves is in accordance with Freundlich adsorption isotherm?
Freundlich adsorption isotherm is given by the expression `x/m = kp^(1/n)` which of the following conclusions can be drawn from this expression.
(i) When `1/n` = 0, the adsorption is independent of pressure.
(ii) When `1/n` = 0, the adsorption is directly proportional to pressure.
(iii) When n = 0, `x/m` vs p graph is a line parallel to x-axis.
(iv) When n = 0, plot of `x/m` vs p is a curve.
Adsorption of gases on the solid surface is generally exothermic because.
Langmuir adsorption isotherm works particularly well.
In the freundilch adsorption isotherm, the value of x/m is 0.4 under a pressure of 0.2 at m calculate the value of the interact
The mass of gas adsorbed, x per unit mass of adsorbed, m was measured at various x pressures p. A graph between `log "x"/"m"` and log p gives a straight line with slope equal to 2 and the intercept equal to 0.4771. The value of `"x"/"m"` at a pressure of 4 atm is ______.
[Given log 3 = 0.4771]
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The x plot of log `x/m` versus log p is shown in the given graph. `x/m` is proportional to ______.