Advertisements
Advertisements
Question
Answer in one sentence:
Write an equation for Freundlich adsorption isotherm.
Solution
Freundlich proposed the following empirical equation for the adsorption of a gas on a solid.
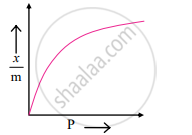
Variation of x/m with P according to Freundlich equation (Adsorption isotherm)
`"x"/"m" = "k""P"^(1//"n")` (n > 1) ....(i)
where,
x = Mass of the gas adsorbed
m = Mass of the adsorbent
`"x"/"m"` = Mass of gas adsorbed per unit mass of adsorbent
P = Equilibrium pressure
k and n are constants which depend on the nature of adsorbate, adsorbent and temperature.
APPEARS IN
RELATED QUESTIONS
What is an adsorption isotherm?
Describe Freundlich adsorption isotherm.
What do you understand by activation of adsorbent? How is it achieved?
In reference to Freundlich adsorption isotherm, write the expression for adsorption of gases on solids in the form of an equation.
Explain graphically Freundlich adsorption isotherm.
The slope and intercept for plot of `log_10 "x"/"m"` against log10P for Freundlich equation are ____________ and ____________ respectively.
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The plot of log `"x"/"m"`· versus log P is shown in the given m graph. `"x"/"m"` is proportional to
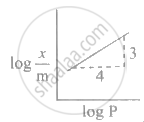
Freundlich's equation for adsorption of gas on solid is represented as ____________.
In Freundlich's adsorption isotherm, when log `("x"/"m")` is plotted against log P, slope of the graph is ____________.
Which of the following curves is in accordance with Freundlich adsorption isotherm?
Freundlich adsorption isotherm is given by the expression `x/m = kp^(1/n)` which of the following conclusions can be drawn from this expression.
(i) When `1/n` = 0, the adsorption is independent of pressure.
(ii) When `1/n` = 0, the adsorption is directly proportional to pressure.
(iii) When n = 0, `x/m` vs p graph is a line parallel to x-axis.
(iv) When n = 0, plot of `x/m` vs p is a curve.
Adsorption of gases on the solid surface is generally exothermic because.
Langmuir adsorption isotherm works particularly well.
Which of the following statement is INCORRECT regarding the adsorption of a gas on the surface of the solid?
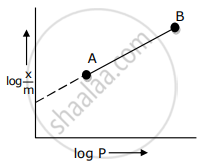
In Freundlich adsorption isotherm, slope of AB line is:
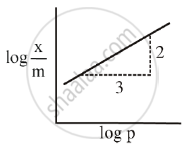
The mass of gas adsorbed, x per unit mass of adsorbed, m was measured at various x pressures p. A graph between `log "x"/"m"` and log p gives a straight line with slope equal to 2 and the intercept equal to 0.4771. The value of `"x"/"m"` at a pressure of 4 atm is ______.
[Given log 3 = 0.4771]
Adsorption of a gas follows Freundlich adsorption isotherm. x is the mass of the gas adsorbed on mass m of the adsorbent. The x plot of log `x/m` versus log p is shown in the given graph. `x/m` is proportional to ______.
For Freundlich adsorption isotherm, a plot of log (x/m) (Y-axis) and log p (x-axis) gives a straight line. The intercept and slope for the line is 0.4771 and 2, respectively. The mass of gas, adsorbed per gram of adsorbent if the initial pressure is 0.04 atm, is ______ × 10-4 g. (log 3 = 0.4771)
