Advertisements
Advertisements
प्रश्न
Give an example of a chemical equation in which two reactants form:
two product
उत्तर
Two product:
\[\ce{NH4OH + HCl->\underset{\text{(two products)}}{NH4Cl + H2O}}\]
APPEARS IN
संबंधित प्रश्न
Translate the following statement into chemical equation and then balance it.
Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
Balance the following equation:
Na + O2  Na2O
Na2O
What are the various ways in which a chemical equation can be made more informative? Give examples to illustrate your answer.
State the various characteristics of chemical reactions.
Why do we need to balance chemical equations?
Write your observation for the following chemical reaction and name the product formed :
When ammonium chloride is heated with sodium hydroxide.
What information does the following chemical equation convey?
Zn + H2SO4 → ZnSO4+ H2
Write the balanced chemical equation of the following reaction.
potassium dichromate + sulphuric acid → potassium sulphate + chromium sulphate + water + oxygen.
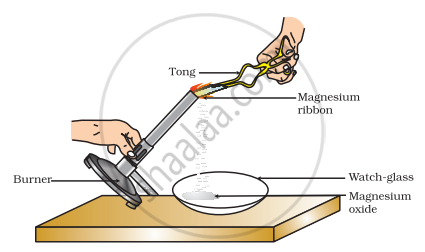
Which of the following is the correct observation of the reaction shown in the above set up?
To balance the following chemical equation the value of x and y should respectively be:
\[\ce{2NaOH + xAl_2O_3->yNaAlO_2 + H_2O}\]
