Advertisements
Advertisements
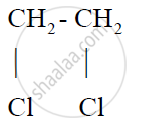
प्रश्न
Give the structural formula for 1, 2-dichloroethane
उत्तर
1, 2-Dichloroethane:
APPEARS IN
संबंधित प्रश्न
Write the next homologue of the following: C2H4
Propane and ethane are ______.
Give the dot diagram of the first member of the alcohol.
Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their (i) physical properties, and (ii) chemical properties.
Study the different conclusions drawn by students of a class on the basis of observations of preserved/available specimens of plants and animals.
I. Potato and sweet potato are analogous organs in plants.
II. Wings of insects and wings of birds are homologous organs in animals.
III. Wings of insects and wings of bats are analogous organs in animals.
IV. Thorns of citrus and tendrils of cucurbita are analogous organs in plants.
The correct conclusions are:
(A) I, and II
(B) II and IV
(C) I and III
(D) III and IV
Copy and complete the following table which relates to three homologus series of hydrocarbons:
| General formula | CnH2n | CnH2n-2 | CnH2n+2 |
| IUPAC name of the homologus series | |||
| Characteristic bond type | Single bonds | ||
| IUPAC name of the first member of the series | |||
| Type of reaction with chlorine | Addition |
Complete the following table for homologous series of Alkenes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Ethene | C2H4 | CH2 = CH2 | 2 | 0 | -102 |
| Propene | C3H6 | CH3–CH = CH2 | 3 | 1 | -48 |
| 1-Butene | C4H8 | CH3–CH2–CH = CH2 | ______ | ______ | -6.5 |
| 1-Pentene | C5H10 | CH3–CH2–CH2–CH = CH2 | ______ | ______ | 30 |
C3H8 belongs to the homologous series of ______.
A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.
- Identify ‘A’ and draw its electron dot structure.
- Give the molecular formulae of any two homologues of ‘A’.
Consider the following molecular formulae of carbon compounds:
(i) CH3COOH (ii) CH3OH (iii) C2H6 (iv) C3H4 (v) C4H8
- Which one of these compounds belongs to homologous series of alcohols?
- Identify the compound having triple bond between carbon-carbon atoms.
- Write the molecular formula of the first member of the homologous series to which CH3COOH belongs.
- Write the general formula of the series to which the compound C4H8 belongs.
