Advertisements
Advertisements
प्रश्न
Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their (i) physical properties, and (ii) chemical properties.
उत्तर
Homologous series of carbon compounds are so called because in such a series of compounds, the same functional group dictates the properties of the carbon compound regardless of the length of the carbon chain. The two consecutive members of a homologous series are CH3OH and C2H5OH (belong to alcohol)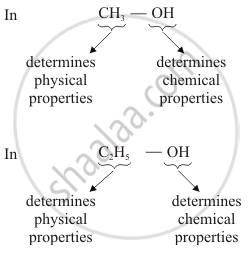
संबंधित प्रश्न
Write the molecular formula of first two members of homologous series having functional group −Br.
Fill in the following blank with suitable word:
Ethene and ethyne are examples of ..... hydrocarbons.
What is the difference in the molecular formula of any two adjacent homologues:
(i) In terms of molecular mass
(ii) In terms of number and kind of atoms per molecule?
The phenomenon in which compounds having different structural formulae have the same molecular formula is called _______.
There are different general molecular formula for all members of the homologous series.
Write a short note.
Homologous series
Observe the structural formula and answer the following questions.

- Write the name of the given hydrocarbon.
- The given hydrocarbon is included in which type of hydrocarbon?
- What is the kind of compounds with the above characteristic structure called?
Study and complete the following table:
| Homologous series | Alkane | Alkyne |
| General formula | CnH2n+2 | 1. ______ |
| IUPAC name | 2. ______ | Ethyne |
| Common name | Marsh gas | 3. ______ |
Consider the carbon compounds having following molecular formula:
(i) C3H6 (ii) C3H8 (iii) C4H6 (iv) C6H6 (v) C6H12
- State the number of double covalent bonds present in C3H8.
- Write the formula of first member of the homologous series to which the carbon compound C4H6 belongs.
- Which one of the above compounds forms a ring structure of carbon atoms?
- Identify, which of the above compounds, is a member of alkane series.
Name the third homologue of alcohols.
