Advertisements
Advertisements
प्रश्न
The phenomenon in which compounds having different structural formulae have the same molecular formula is called _______.
विकल्प
structural isomerism
catenation
homologous
functional group
उत्तर
The phenomenon in which compounds having different structural formulae have the same molecular formula is called structural isomerism.
APPEARS IN
संबंधित प्रश्न
Write the next homologue of the following: C2H4
Write the name and formula of the 2nd member of homologous series having general formula CnH2n.
Write the molecular formula of first two members of homologous series having functional group -Cl.
The following vegetables are kept in a basket :
Potato, Tomato, Radish, Brinjal, Carrot, Bottle-gourd
Which two of these vegetables correctly represent the homologous structures?
(A) Carrot and Tomato
(B) Potato and Brinjal
(C) Radish and Carrot
(D) Radish and Bottle-gourd
Give the structural formula for 1, 2-dichloroethane
Fill in the following blank with suitable word:
Ethene and ethyne are examples of ..... hydrocarbons.
Fill in the following blank with suitable word:
Carbon compounds have usually ... melting points and boiling points because they are ...... in nature.
Write the molecular formula of the third member of the homologous series of carbon compounds with general formula CnHO2n+1OH.
The molecular formula of a homologue of butane is:
(a) C4H8
(b) C3H6
(c) C4H6
(d) C3H8
Propane and ethane are ______.
Give the dot diagram of the first member of the alcohol.
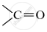 .
.
Write the name and molecular formula of the first member of the homologous series of alkynes.
Write the name and formula of the fourth member of the following homologous series:
Alkyne
The general formula of Alkane is _________________
While going in an increasing order there is a rise in the molecular mass of the consecutive members of the homologous series by _______.
Cyclohexane : Cyclic hydrocarbon : : Isobutylene : _______
Complete the following chart by using examples given in brackets.
(isobutylene, cyclohexane, propane, cyclohexene, cyclopentane, benzene, propyne, isobutane, propene)
| Straight chain hydrocarbons | Branched chain hydrocarbons | Cyclic hydrocarbons |
Which of the following does not belong to the same homologous series?
Consider the following molecular formulae of carbon compounds:
(i) CH3COOH (ii) CH3OH (iii) C2H6 (iv) C3H4 (v) C4H8
- Which one of these compounds belongs to homologous series of alcohols?
- Identify the compound having triple bond between carbon-carbon atoms.
- Write the molecular formula of the first member of the homologous series to which CH3COOH belongs.
- Write the general formula of the series to which the compound C4H8 belongs.
