Advertisements
Advertisements
प्रश्न
The phenomenon in which compounds having different structural formulae have the same molecular formula is called _______.
पर्याय
structural isomerism
catenation
homologous
functional group
उत्तर
The phenomenon in which compounds having different structural formulae have the same molecular formula is called structural isomerism.
APPEARS IN
संबंधित प्रश्न
What is meant by homologous series of carbon compounds?
Write the name and formula of the 2nd member of homologous series having general formula CnH2n + 2.
What is the difference between two consecutive homologues:
(1) in terms of molecular mass?
(2) in terms of number and kind of atoms per molecule?
Give the molecular formula of one homologue of each of the following:
C2H6
By how many carbon atoms and hydrogen atoms do any two adjacent homologues differ?
The molecular formula of an organic compound is C18H36. Name its homologous series.
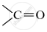 .
.
Give the names of the first four members of the homologous series of
alkynes.
Give three points to differentiate between saturated and unsaturated
hydrocarbons.
The general formula of Alkane is _________________
The general molecular formula for the homologous series of alkynes is _______.
Saturated hydrocarbon : Single bond : : Unsaturated hydrocarbon : _______
There are different general molecular formula for all members of the homologous series.
Write a short note.
Homologous series
Complete the following table for homologous series of alcohols.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methanol | CH4O | CH3-OH | 1 | 1 | 63 |
| Ethanol | C2H6O | CH3–CH2-OH | 2 | 2 | 78 |
| Propanol | C3H8O | CH3–CH2–CH2-OH | ______ | ______ | 97 |
| Butanol | C4H10O | CH3–CH2–CH2–CH2–OH | ______ | ______ | 118 |
The first member of alkyne homologous series is
Consider the following molecular formulae of carbon compounds:
(i) CH3COOH (ii) CH3OH (iii) C2H6 (iv) C3H4 (v) C4H8
- Which one of these compounds belongs to homologous series of alcohols?
- Identify the compound having triple bond between carbon-carbon atoms.
- Write the molecular formula of the first member of the homologous series to which CH3COOH belongs.
- Write the general formula of the series to which the compound C4H8 belongs.
