Advertisements
Advertisements
प्रश्न
Give the main experimental points only to demonstrate that candle-gains weight on burning.
उत्तर
Candle gains weight on burning
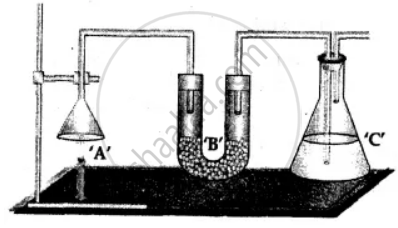
1. Weight: The complete apparatus as shown above which includes -
- Unlit candle ‘A’.
- U tube ‘B’ containing anhydrous CaCl2.
- Conical flask ‘C’ containing cone. KOH soln.
Total wt. = a gms.
The candle ‘A’ is then lit and the products obtained on burning are allowed to be absorbed in U tube ‘B’ and conical flask ‘C’.
2. Re-weigh: The complete apparatus as shown above after the candle has burnt – for a known period of time.
Total wt. = b gms.
Result:
- ‘b’ gms. is greater than ‘a’ gms.
- weight of apparatus after absorbtion
- of products is greater than the original weight of the apparatus.
Conclusion: Candle gains weight due to mass of oxygen of the air which has combined with ‘carbon’ and ‘hydrogen’ of the candle [CxHy] producing .
- Water vapour [absorbed by anhydrous CaCl2]
- Carbon dioxide [absorbed by cone. KOH soln].
APPEARS IN
संबंधित प्रश्न
A metal highly resistant to rusting.
The process by which oxidation of food in our body takes place is
Write ‘True’ or ‘False’ in front of following statement.
Nitrogen is a gaseous non-metal essential for respiration.
How will you prove that oxide formed by burning sodium is basic in nature?
Write the balanced chemical equation of the manganese dioxide.
Describe simple experiments to show the presence of -
oxygen and nitrogen component in air using a bell jar
Describe simple experiments to show the presence of -
carbon dioxide in air using a test tube with outlets containing lime water.
Describe simple experiments to show the presence of -
water vapour in air using a glass tumbler and ice.
In the laboratory preparation of oxygen from hydrogen peroxide answer the following:
Name the catalyst used in the preparation and state it’s function.
Give reason for the following:
Combustion and respiration show similarity.
