Advertisements
Advertisements
प्रश्न
Give the main experimental points only to demonstrate that magnesium.
उत्तर
Experiment to show that magnesium gains weight on burning:
Take dry crucible with lid. Put a small piece of magnesium in it and cover the lid. Find its weight with lid covering it. Let it be 50 gms.
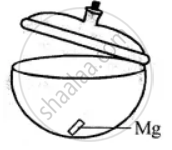
Now heat the crucible along with magnesium and lid just opened for a while. Taking care that no ash comes out. Let it cool covering the lid and products formed weight it again. The weight now will be more. Let it be 50.82 gms, oxygen combined is 50.82 – 50 = 0.82 gms this shows that something is added to magnesium, which has increased its weight. This 0.82 gm is the weight of oxygen added to magnesium.
2Mg + O2 → 2MgO
Hence magnesium gains weight on burning.
APPEARS IN
संबंधित प्रश्न
What do you observe when Magnesium is heated and then tested with moist blue and red litmus – paper?
What happens when Iron burns in oxygen ? Also give the balanced chemical equation for the reaction.
Write ‘True’ or ‘False’ in front of following statement.
The rust formed on the surface of iron easily crumbles.
What is rusting?
State four ways of preventing rusting.
Oxygen of the air is utilised for ‘combustiop’ and for ‘respiration’. Compare the two with a suitable examples.
Identify the acidic oxides responsible for acid rain. State how their presence results in formation of acid rain. Give a reason why acid rain damages heritage buildings.
In the laboratory preparation of oxygen from hydrogen peroxide answer the following:
State the word equation for the reaction involving the above preparation of oxygen.
Distinguish between:
Combustion and Respiration
Distinguish between:
Combustion & Rusting with suitable word equations only?
