Advertisements
Advertisements
प्रश्न
How is ethyne prepared in the laboratory?
उत्तर
Ethyne Is prepared by the reaction of calcium carbide with water
\[\ce{CaC2 + 2H2O -> C2H2 + Ca(OH)2}\]
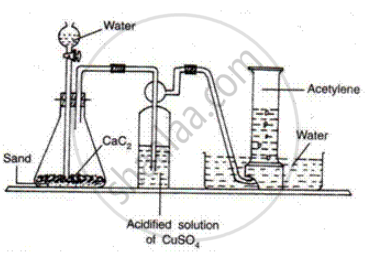
APPEARS IN
संबंधित प्रश्न
A student puts a drop of reaction mixture of a saponification reaction first a blue litmus paper and then on a red litmus paper. He may observe that:
(a) There is no change in the blue litmus paper and the red litmus paper turns white.
(b) There is no change in the red litmus paper and the blue litmus paper turns red.
(c) There is no change in the blue litmus paper and the red litmus paper turns blue.
(d) No change in colour is observed in both the litmus papers
Complete the following chemical equations : C2H5OH`("Conc."H_2SO_4)/(443K)`>
Give the common names and IUPAC names of the following compounds of HCOOH.
Fill in the following blank with suitable word:
The next higher homologue of ethanol is ...............
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
Name the products formed and give appropriate chemical equations for the following:
sodium reacting with ethyl alcohol.
Acetic acid is a typical acid. Write one equation in case of its reactions with a carbonate.
On adding acetic acid to sodium hydrogen carbonate in a test tube, a student observes
(A) no reaction
(B) a colourless gas with pungent smell
(C) bubbles of a colourless and odourless gas
(D) a strong smell of vinegar
Ester is a sweet-smelling compound.
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Write a chemical equation for the reaction.
