Advertisements
Advertisements
प्रश्न
How is sodium metal extracted? Explain with the help of equation of the reaction involved.
उत्तर
Sodium is extracted by electrolytic reduction of molten sodium chloride. On passing electricity through molten sodium chloride, decomposition occurs and sodium metal and chlorine gas are formed.
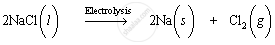
Molten sodium chloride contains sodium and chloride ions. The reactions that occur during electrolysis are:
1. The cathode produces electrons to reduce sodium ions to sodium atoms (or sodium metal) by acting as reducing agent. Sodium ions are cations; therefore, they are attracted to the negatively-charged cathode and get deposited there.
Cathode: 
Chloride ions are anions; therefore they are attracted to the positively-charged anode. These chloride ions are oxidised to chlorine gas. Chlorine gas is produced at the anode.
Anode: 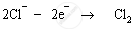
APPEARS IN
संबंधित प्रश्न
Name the following: Main ore of aluminium.
Name two metals which are found in nature mainly in the free state (as metallic elements).
What iron compound is present in haematite ore? Also write its chemical formula.
Which of the following is an iron ore?
(a) cinnabar
(b) calamine
(c) haematite
(d) rock salt
The two metals which are extracted by means of electrolytic reduction of their molten salts are:
(a) magnesium and manganese
(b) iron and aluminium
(c) zinc and magnesium
(d) magnesium and aluminium
Some metallic oxides can be reduced by hydrogen, carbon and carbon monoxide and some cannot. Explain.
Define roasting.
In Electrolytic reduction of alumina, Anode : _______ : : Cathode : Graphite lining
Answer the questions on the following passage.
The minerals from which the metal can be separated economically are called ores. Ores contain many types of impurities such as soil, sand and rocky substances along with metal compounds. These impurities are called gangue.
Metals can be extracted from their ores by means of various methods of separation. The process of extraction of metal in a pure state from the ores is also a part of metallurgy.
Ores are taken out from the mines and the gangue is usually separated from the ore at the site itself by various methods. Then the ores are carried out to the place where metals are produced. Here metals are extracted in pure form. Then metals are further purified by different methods of purification. This entire process is called metallurgy. Most metals being reactive do not occur in nature in the free state but are found in combined state as their salts such as oxides, carbonates, sulphides, and nitrates. however, the most unreactive metals that are not affected by air, water and other natural factors like silver, gold, platinum, generally occur in a free state. The compounds of metals that occur in nature along with the impurities are called minerals.
- What are ores?
- Which processes are involved in the branch of metallurgy? What is metallurgy?
- Which metals are found in a free state?
- In what forms are metals found in combined state?
- What is gangue?
Which of the following metals exist in their native state in nature?
- Cu
- Au
- Zn
- Ag
