Advertisements
Advertisements
प्रश्न
Hydrogen is a good reducing agent: What do you understand by the above statement? Explain with the help of copper oxide as an example.
उत्तर
Hydrogen acts as a good reducing agent means, when hydrogen gas is passed over hot metallic oxides of copper, lead, iron, etc. it removes oxygen from them and thus reduces them to their corresponding metal.
Let us consider the following example, in each of which metallic oxide react with hydrogen. Metallic oxide act as oxidising agents and hydrogen acts as a reducing agents.
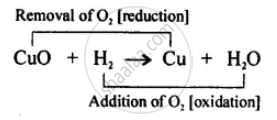
APPEARS IN
संबंधित प्रश्न
Complete and balance the following equation:
H2+____________→ 2HCI
Multiple Choice Question
Hydrogen is
FILL IN THE BLANK
Hydrogen is present abundantly in the ................
Write the balanced equation and give your observation when the following metal reacts:
Calcium with cold water
Using a burning candle and a jar of hydrogen – how would you prove experimentally that Hydrogen is a combustible gas.
Using a burning candle and a jar of hydrogen – how would you prove experimentally that Hydrogen does not support combustion.
Write correctly the balanced equation for the following:
‘When zinc filings are added to a concentrated solution of sodium hydroxide’.
Write down the “word equation” for the following reaction:
sodium hydroxide solution + zinc → ?
How would you obtain ‘hydrogen from sodium hydroxide’ solution other than by electrolysis?
What do the following symbols represent : 2H and H2.
