Advertisements
Advertisements
Question
Hydrogen is a good reducing agent: What do you understand by the above statement? Explain with the help of copper oxide as an example.
Solution
Hydrogen acts as a good reducing agent means, when hydrogen gas is passed over hot metallic oxides of copper, lead, iron, etc. it removes oxygen from them and thus reduces them to their corresponding metal.
Let us consider the following example, in each of which metallic oxide react with hydrogen. Metallic oxide act as oxidising agents and hydrogen acts as a reducing agents.
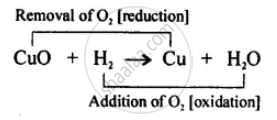
APPEARS IN
RELATED QUESTIONS
Indicate which of the following statement is true and which is false:
Hydrogen is combustible as well as a supporter of combustion.
MULTIPLE CHOICE QUESTIONS
The name hydrogen was given by
- Cavendish
- Lavoisier
- Haber
- none of these
MULTIPLE CHOICE QUESTIONS
Which is the lightest of all elements?
- hydrogen
- helium
- lithium
- none of these
FILL IN THE BLANK
In nature, hydrogen occurs as a .................. molecule represented as H2
FILL IN THE BLANK
Ammonia is used to make ................
Write the balanced equation and give your observation when the following metal reacts:
Calcium with cold water
Give a test to differentiate between two gas jars – one containing pure hydrogen and the other hydrogen-air mixture.
Select the correct options for the following statement:
The gas which has now replaced hydrogen in air balloons is ___.
Explain the following:
Two jars of H2 are collected – “one burns quietly and the other does not”.
Describe one chemical test applied to the following gases, which would enable you to distinguish between them :‘carbon monoxide and hydrogen’.
