Advertisements
Advertisements
प्रश्न
Identify the α-carbon in the following species and give the total number of α-hydrogens.
\[\ce{CH2 = CH - CH2 - CH3}\]
उत्तर
\[\ce{\overset{1}{C}H2 = \overset{2}{C}H - \overset{3}{\underset{\alpha}{C}}H2 - \overset{4}{C}H3}\]
The C-3 carbon atom, that is, the C-atom next to \[\ce{(H2C = CH-)}\] is an α-C atom.
Thus, the structure contains 2 α-H atoms.
APPEARS IN
संबंधित प्रश्न
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH_3 - CH_2 - \overset{⊕}{C}H-CH_2 - CH_3}\]
Draw a resonance structure of the following:
Benzaldehyde
Write true or false. Correct the false statement.
Heterolytic fission results in the formation of free radicals.
Draw all the no-bond resonance structures of isopropyl carbocation.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
Choose the correct option.
The geometry of a carbocation is ______.
Choose the correct option.
The homologous series of alcohols has general molecular formula ______.
Which of the following compound is highly reactive towards HCN?
Which of the following is the strongest nucleophile?
Which among the following is a set of nucleophiles?
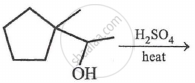
The best reagent for the following conversion is:
The overlap of σ-p orbitals is called ____________.
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
Resonance is NOT exhibited by ____________.
Which of the following statements is not correct?
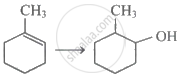
The most probable product in the reaction given below is:
Identify the functional group that has an electron-donating inductive effect.
Arrange the following free radicals in order of decreasing stability.
- Methyl
- Vinyl
- Allyl
- Benzyl
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
