Advertisements
Advertisements
प्रश्न
Draw a resonance structure of the following:
Benzaldehyde
उत्तर
Resonance structures of benzaldehyde:

APPEARS IN
संबंधित प्रश्न
Find out the most stable species from the following. Justify
`dot"CH","CH"_3-dot"CH" - "CH"_3,` \[\begin{array}{cc}\ce{CH3 -\dot{C} - CH3}\\
|\phantom{.}\\\phantom{..}\ce{CH3}\end{array}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH_3 - CH_2 - \overset{⊕}{C}H-CH_2 - CH_3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
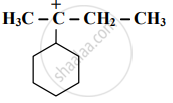
Identify the α-carbon in the following species and give the total number of α-hydrogens.
\[\ce{CH2 = CH - CH2 - CH3}\]
Draw a resonance structure of the following:
Phenol
Distinguish between Homolysis and heterolysis.
Write true or false. Correct the false statement.
Homolytic fission involves the unsymmetrical breaking of a covalent bond.
The correct IUPAC name of the compound 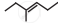 is ______.
is ______.
Predict the order of reactivity of the following compounds by SNl reaction mechanism.
\[\ce{\underset{\text{(I)}}{C6H5CH(C6H5)Cl}}\]
\[\ce{\underset{\text{(II)}}{C6H5CH2Cl}}\]
\[\ce{\underset{\text{(III)}}{C6H5C(CH3)(C6H5)Cl}}\]
Which of the following compound is highly reactive towards HCN?
Which of the following is the strongest nucleophile?
Which among the following is a set of nucleophiles?
Identify the reagent used in the following reaction:
\[\ce{CH3 - CH2 - Br ->[?] CH3 - CH2 - OH}\]
The most unstable free radical among the following is:
The overlap of σ-p orbitals is called ____________.
Which of the following is the most unstable carbocation?
Which of the following is NOT an electrophile?
How many pi bonds and sigma bonds are present in following molecule?
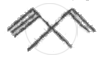
Which of the following statements is not correct?
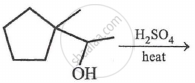
The most probable product in the reaction given below is:
Arrange the following free radicals in order of decreasing stability.
- Methyl
- Vinyl
- Allyl
- Benzyl
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH2 }\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogens.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
