Advertisements
Advertisements
प्रश्न
Write true or false. Correct the false statement.
Homolytic fission involves the unsymmetrical breaking of a covalent bond.
विकल्प
True
False
उत्तर
Homolytic fission involves the unsymmetrical breaking of a covalent bond.- False.
Correct Statement:
Homolytic fission involves the symmetrical breaking of a covalent bond.
APPEARS IN
संबंधित प्रश्न
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH_3 - CH_2 - \overset{⊕}{C}H-CH_2 - CH_3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
Draw a resonance structure of the following:
Buta-1,3-diene
Distinguish between Inductive effect and resonance effect.
Write true or false. Correct the false statement.
Free radicals are negatively charged species.
Choose the correct option.
The geometry of a carbocation is ______.
Choose the correct option.
The delocalization of electrons due to overlap between p orbital and sigma bond is called _______.
Predict the order of reactivity of the following compounds by SNl reaction mechanism.
\[\ce{\underset{\text{(I)}}{C6H5CH(C6H5)Cl}}\]
\[\ce{\underset{\text{(II)}}{C6H5CH2Cl}}\]
\[\ce{\underset{\text{(III)}}{C6H5C(CH3)(C6H5)Cl}}\]
Which of the following compound is highly reactive towards HCN?
Identify the reagent used in the following reaction:
\[\ce{CH3 - CH2 - Br ->[?] CH3 - CH2 - OH}\]
The most unstable free radical among the following is:
Which of the following statements is not correct?
The most probable product in the reaction given below is:
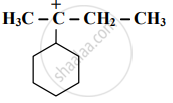
Which element among the following does form pπ - pπ multiple bonds?
Arrange the following free radicals in order of decreasing stability.
- Methyl
- Vinyl
- Allyl
- Benzyl
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogens.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
