Advertisements
Advertisements
प्रश्न
Draw a resonance structure of the following:
Benzaldehyde
उत्तर
Resonance structures of benzaldehyde:

APPEARS IN
संबंधित प्रश्न
Find out the most stable species from the following. Justify.
`bar"C""H"_3, bar"C""H"_2"Br", bar"C""Br"_3`
Identify the α - carbons in the following species and give the total number of α-hydrogen.
Draw a resonance structure of the following:
Phenol
Distinguish between Inductive effect and resonance effect.
Write true or false. Correct the false statement.
Heterolytic fission results in the formation of free radicals.
Write true or false. Correct the false statement.
Aniline is a heterocyclic compound.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
Choose the correct option.
The delocalization of electrons due to overlap between p orbital and sigma bond is called _______.
Which among the following is a set of nucleophiles?
The best reagent for the following conversion is:
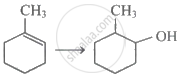
Which of the following shows positive resonance (+R) effect?
IUPAC name of ![]() is ______.
is ______.
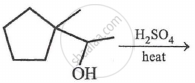
The most probable product in the reaction given below is:
Identify the functional group that has an electron-donating inductive effect.
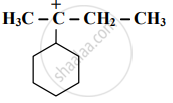
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH2 }\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
CH2 = CH - CH2 - CH3
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH3}\]
