Advertisements
Advertisements
प्रश्न
Draw a resonance structure of the following:
Buta-1,3-diene
उत्तर
Resonance structures of buta-1,3-diene:

APPEARS IN
संबंधित प्रश्न
Find out the most stable species from the following. Justify
`dot"CH","CH"_3-dot"CH" - "CH"_3,` \[\begin{array}{cc}\ce{CH3 -\dot{C} - CH3}\\
|\phantom{.}\\\phantom{..}\ce{CH3}\end{array}\]
Find out the most stable species from the following. Justify.
\[\ce{\overset{+}{C}H3, \overset{+}{C}H2Cl, \overset{+}{C}Cl3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH_3 - CH_2 - \overset{⊕}{C}H-CH_2 - CH_3}\]
Draw a resonance structure of the following:
Acetate ion
Distinguish between Electrophile and nucleophile.
Distinguish between Homolysis and heterolysis.
Write true or false. Correct the false statement.
Free radicals are negatively charged species.
Draw all the no-bond resonance structures of isopropyl carbocation.
Which of the following is NOT an electrophile?
Which of the following is the strongest nucleophile?
Which of the following statements is INCORRECT about hyperconjugation?
Identify the reagent used in the following reaction:
\[\ce{CH3 - CH2 - Br ->[?] CH3 - CH2 - OH}\]
Which of the following alkyl groups shows maximum positive inductive effect?
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
Which of the following is NOT an electrophile?
The most probable product in the reaction given below is:
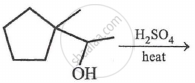
Identify the functional group that has an electron-donating inductive effect.
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH2 }\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogens.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
