Advertisements
Advertisements
प्रश्न
In an experiment to study the properties of acetic acid a student takes about 2 mL of acetic acid in a dry test tube. He adds about 2 mL of water to it and shakes the test tube well. He is likely to observe that:
(A) the acetic acid dissolves readily in water
(B) the solution becomes light orange
(C) water floats over the surface of acetic acid
(D) acetic acid floats over the surface of water
उत्तर
(A)
The acetic acid dissolves readily in water.
APPEARS IN
संबंधित प्रश्न
What are esters ?
Complete the following chemical equations:CH3COOC2H5+NaOH→
Complete the following chemical equations: CH3COOH+NaHCO3→
Name one chemical compound which can be used to distinguish between ethanol and ethanoic acid.
What happens when propanoic acid is warmed with methanol in the presence of a few drops of concentrated sulphuric acid? Write equation of the reaction involved.
How would you distinguish between ethanol and ethanoic acid by chemical test?
If you are asked to report your observations about the following two properties of acetic acid, what would you report?
(i) Odour
(ii) Effect on litmus
Draw the structure formula of ethyne.
Convert ethane to acetic acid.
Observe the diagram given below and answer the questions:
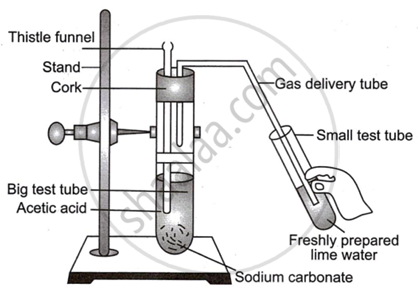
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
