Advertisements
Advertisements
प्रश्न
Isotherms of carbon dioxide gas are shown in figure. Mark a path for changing gas into liquid such that only one phase (i.e., either a gas or a liquid) exists at any time during the change. Explain how the temperature, volume and pressure should be changed to carry out the change.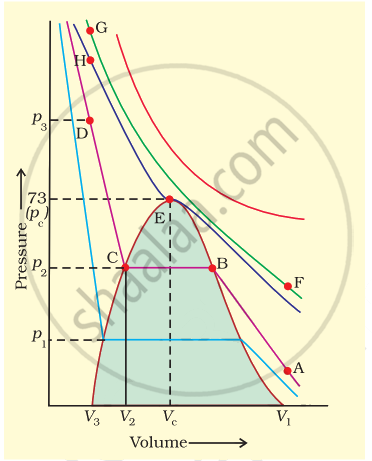
उत्तर
In isotherm of carbon dioxide, it is possible to change a gas into liquid or a liquid into gas by a process in which always a single phase is present.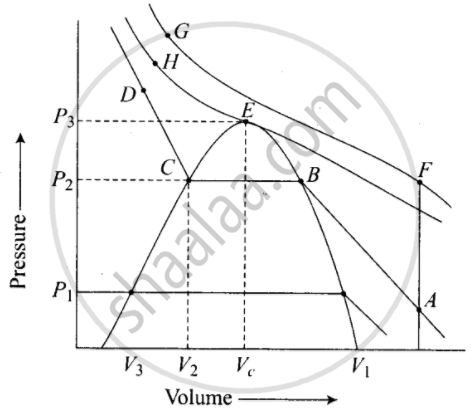
If we move vertically from point \[\ce{A}\] to \[\ce{F}\] by increasing the temperature, then we can reach the point G by compressing the gas at constant temperature along this (isotherm at 31.1°C). Now we can move vertically downwards to \[\ce{D}\] by lowering the temperature. As soon as we cross point \[\ce{H}\] on critical isotherm, we get liquid. If process is carried out at critical temperature, substance always remains in one phase. Hence the path for the change is \[\ce{A → F → G → H → D}\]
APPEARS IN
संबंधित प्रश्न
The value of the universal gas constant depends upon
Which of the following diagrams correctly describes the behaviour of a fixed mass of an ideal gas? (T is measured in K)
25 g of each of the following gases are taken at 27°C and 600 mm Hg pressure. Which of these will have the least volume?
In what way real gases differ from ideal gases.
Explain whether a gas approaches ideal behavior or deviates from ideal behaviour if more gas is introduced into the same volume and at the same temperature.
Which of the following gases would you expect to deviate from ideal behavior under conditions of low-temperature F2, Cl2, or Br2? Explain.
Write the Van der Waals equation for a real gas. Explain the correction term for pressure and volume.
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in Figure. Which of the following order of pressure is correct for this gas?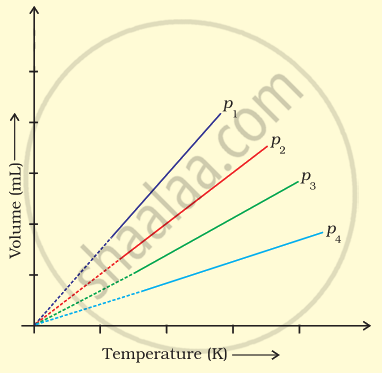
Pressure versus volume graph for a real gas and an ideal gas are shown in figure. Answer the following questions on the basis of this graph.
(i) Interpret the behaviour of real gas with respect to ideal gas at low pressure.
(ii) Interpret the behaviour of real gas with respect to ideal gas at high pressure.
(iii) Mark the pressure and volume by drawing a line at the point where real gas behaves as an ideal gas.
Assertion (A): At constant temperature, pV vs V plot for real gases is not a straight line.
Reason (R): At high pressure all gases have \[\ce{Z}\] > 1 but at intermediate pressure most gases have \[\ce{Z}\] < 1.
