Advertisements
Advertisements
प्रश्न
KMnO4 is coloured due to ______.
विकल्प
d-d transitions
charge transfer from ligand to metal
unpaired electrons in d orbital of Mn
charge transfer from metal to ligand
उत्तर
KMnO4 is coloured due to charge transfer from ligand to metal.
Explanation:
The Mn atom in KMnO4 has a +7 oxidation state with electron configuration [Ar]3d04s0. Since no unpaired electrons are present, d−d transitions are not possible. The molecule should, therefore, be colourless.
Its intense purple due to `"L"→"M"` (ligand to metal) charge transfer 2p(L) of O to 3d(M) of Mn.
APPEARS IN
संबंधित प्रश्न
Complete the following equations:
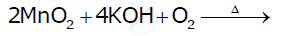
Complete the following equations: Cr2O72- + 14H+ + 6I →
Indicate the steps in the preparation of KMnO4 from pyrolusite ore.
Write the ionic equation showing the oxidation of Fe(II) salt by acidified dichromate solutions.
On addition of small amount of \[\ce{KMnO4}\] to concentrated \[\ce{H2SO4}\], a green oily compound is obtained which is highly explosive in nature. Identify the compound from the following.
Potassium dichromate when heated with concentrated sulphuric acid and a soluble chloride, gives brown-red vapours of ______.
Complete the reaction mentioning all the products formed:
\[\ce{2KMnO4 ->[\Delta]}\]
Which of the following ions will have a magnetic moment value of 1.73 BM.
\[\ce{Sc^3+, Ti^3+, Ti^2+, Cu^2+, Zn^2+}\]
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
