Advertisements
Advertisements
प्रश्न
Name the electrode at which aluminium metal is produced.
उत्तर
Aluminium metal is produced at cathode. During electrolysis, cathode produces electrons to reduce aluminium ions into aluminium atoms (or aluminium metal) by acting as reducing agent. Aluminium ions are cations; therefore, they get attracted to negatively charged cathode and get deposited there.
Cathode: 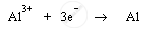
APPEARS IN
संबंधित प्रश्न
In the extraction of aluminium: Name the process of concentration of bauxite.
Name the gas produced when calamine ore is calcined.
Rock salt is an ore of one of the following metals. This metal is:
(a) Mn
(b) Na
(c) Fe
(d) Cu
Give the principles of the hydrolytic method.
Which one of the following is not true of metal :
Metals are malleable and ductile
Answer the following question :
What is the difference between calcination and roasting.
Name the following:
A steel grey coloured solid non-metal.
Extraction of moderately reactive elements is done by _______ and _______ method.
Gold and silver are active metals.
Explain the following reaction with the balanced equation.
Sodium aluminate reacts with water
