Advertisements
Advertisements
प्रश्न
How is aluminium metal extracted? Explain with the help of an equation.
उत्तर
Aluminium is extracted by electrolytic reduction of its molten oxide. Aluminium is a highly reactive metal and it is placed at the top of the reactivity series. This cannot be reduced by other reducing agents such as coke, carbon monoxide etc.
On passing electricity through molten aluminium oxide, decomposition reaction occurs and formation of aluminium metal and oxygen gas takes place.

Molten aluminium oxide consists of aluminium and oxide ions. The reactions that occur during electrolysis are:
1. Cathode produces electrons to reduce aluminium ions to aluminium atoms (or aluminium metal) by acting as reducing agent. Aluminium ions are cations; therefore, they get attracted to negatively charged cathode and get deposited there.
Cathode: 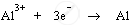
xide ions are anions; therefore, they get attracted to positively charged anode. These oxide ions are oxidised to oxygen gas. Oxygen gas is produced at anode.
Anode: 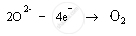
APPEARS IN
संबंधित प्रश्न
Name the most abundant metal in the earth's crust.
Name one metal which is extracted by electrolytic reduction.
Name the electrode at which aluminium metal is produced.
Which of the following pair of metals exists in their native state in nature?
(a) Ag and Hg
(b) Ag and Zn
(c) Au and Hg
(d) Au and Ag
Metals X and Y can be recovered from the anode mud left behind after the electrolytic refining of copper metal. The coins made of metal X look new even after several years of use but the coins made of metal Y lose their shine gradually and get blackened soon. When metal X is alloyed with a small amount of metal Y, it becomes hard and hence suitable for making ornaments. What are metals X and Y? Also state the colour of metal X.
Give the chemical formula of :
Cryolite
Which one of the following is not true of metal :
Metals are malleable and ductile
Atomic number of aluminium is _______ and its electronic configuration is _______.
Explain the following reaction with the balanced equation.
Sodium aluminate reacts with water
Iqbal treated a lustrous, divalent element M with sodium hydroxide. He observed the formation of bubbles in reaction mixture. He made the same observations when this element was treated with hydrochloric acid. Suggest how can he identify the produced gas. Write chemical equations for both the reactions.
