Advertisements
Advertisements
प्रश्न
How is aluminium metal extracted? Explain with the help of an equation.
उत्तर
Aluminium is extracted by electrolytic reduction of its molten oxide. Aluminium is a highly reactive metal and it is placed at the top of the reactivity series. This cannot be reduced by other reducing agents such as coke, carbon monoxide etc.
On passing electricity through molten aluminium oxide, decomposition reaction occurs and formation of aluminium metal and oxygen gas takes place.

Molten aluminium oxide consists of aluminium and oxide ions. The reactions that occur during electrolysis are:
1. Cathode produces electrons to reduce aluminium ions to aluminium atoms (or aluminium metal) by acting as reducing agent. Aluminium ions are cations; therefore, they get attracted to negatively charged cathode and get deposited there.
Cathode: 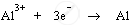
xide ions are anions; therefore, they get attracted to positively charged anode. These oxide ions are oxidised to oxygen gas. Oxygen gas is produced at anode.
Anode: 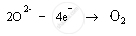
APPEARS IN
संबंधित प्रश्न
In the extraction of aluminium Write the cathode reaction in electrolytic reduction of alumina.
In the extraction of aluminium: Why is it necessary to replace anodes time to time?
Explain givem equation, what happens when a mixture of Cu2O and Cu2S is heated?
What iron compound is present in haematite ore? Also write its chemical formula.
The two metals which are extracted by means of electrolytic reduction of their molten salts are:
(a) magnesium and manganese
(b) iron and aluminium
(c) zinc and magnesium
(d) magnesium and aluminium
Name the anode, the cathode and the electrolyte used in the electrolytic refining of impure copper.
What is meant by refining of metals? Name the three common methods used for refining.
Name the process used for the enrichment of sulphide ore.
In Electrolytic reduction of alumina, Anode : _______ : : Cathode : Graphite lining
The process in which a carbonate ore is heated strongly in the absence of air to convert it into metal oxide is called ____________.
