Advertisements
Advertisements
प्रश्न
Name the electrode at which aluminium metal is produced.
उत्तर
Aluminium metal is produced at cathode. During electrolysis, cathode produces electrons to reduce aluminium ions into aluminium atoms (or aluminium metal) by acting as reducing agent. Aluminium ions are cations; therefore, they get attracted to negatively charged cathode and get deposited there.
Cathode: 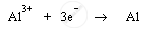
APPEARS IN
संबंधित प्रश्न
In the extraction of aluminium: Name the process of concentration of bauxite.
In the extraction of aluminium: Write the function and formula of cryolite in the extraction of aluminium.
Silver metal does not combine easily with oxygen but silver jewellery tarnishes after some time. How?
Name one metal which is extracted by electrolytic reduction.
The metal which can be extracted from pyrolusite ore is:
(a) mercury
(b) manganese
(c) aluminium
(d) magnesium
The following questions relate to the extraction of aluminium by electrolysis.
Explain why it is necessary to renew the anode periodically.
What is the difference between calcination and roasting?
If Cu, Fe, Zn, Al elements are arranged in increasing order of their reactivity then the correct order would be which of the following?
Gold and silver are active metals.
In extraction of copper, the flux used is ____________.
