Advertisements
Advertisements
प्रश्न
Phenol can be distinguished from ethanol by the reactions with:
(i) \[\ce{Br2/water}\]
(ii) \[\ce{Na}\]
(iii) Neutral \[\ce{FeCl3}\]
(iv) All the above
उत्तर
(i) \[\ce{Br2/water}\]
(iii) Neutral \[\ce{FeCl3}\]
Explanation:
Ethanol does not give any reaction with neutral \[\ce{FeCl3}\] solution while phenol gives violet colour-neutral \[\ce{FeCl3}\]. When phenol is treated with bromine water, 2, 4, 6-tribromophenol is formed as white precipitate. Ethanol does not react with bromine water.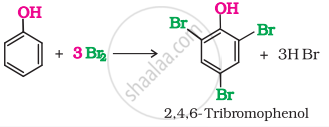
APPEARS IN
संबंधित प्रश्न
How do you convert the following :
Ethanol to Propan-2-ol
Give simple chemical test to distinguish between Ethanol and Phenol.
If ethanol dissolves in water, then which of the following would be observed:
Arrange the following compounds in increasing order of acidity and give a suitable explanation.
Phenol, o-nitrophenol, o-cresol
Dipole moment of phenol is smaller than that of methanol. Why?
The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Match the items of column I with items of column II.
| Column I | Column II | |
| (i) | Antifreeze used in car engine | (a) Neutral ferric chloride |
| (ii) | Solvent used in perfumes | (b) Glycerol |
| (iii) | Starting material for picric acid | (c) Methanol |
| (iv) | Wood spirit | (d) Phenol |
| (v) | Reagent used for detection of phenolic group | (e) Ethleneglycol |
| (vi) | By product of soap industry used in cosmetics | (f) Ethanol |
Alcoholic fermentation is brought about by the action of
Write chemical reactions of following reagents on methoxyethane:
dilute H2SO4
Give IUPAC names of the following compounds:
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH - CH2-OH }\\
|\phantom{......}|\phantom{......}|\phantom{.......}\\
\ce{Cl}\phantom{....}\ce{CH3}\phantom{...}\ce{CH3}\phantom{.....}
\end{array}\]
