Advertisements
Advertisements
प्रश्न
Potassium nitrate on strong heating decomposes as under :
2KNO3 → 2KNO2 + O2
Calculate : Weight of oxygen formed when 5.05g of potassium nitrate decomposes completely.
(K = 39, 0 = 16, N = 14)
उत्तर
Given equation is: 2KNO3 → 2KNO2 + O2
Molecular mass of KNO3 is : (Atomic mass of K + Atomic mass of N + Atomic mass of O) = (39 + 14 + 16 x 3 ) = 101
Molecular mass of of KNO2 = (39 + 14 + 16 x 2) = 85
Now, decomposition of 101g of KNO3 yield = 16g of O2
So, decomposition of 5.05 g of KNO3 will yield = 16 x 5 .05 / 101 = 0.8 g
Hence, when 5.05g of potassium nitrate decomposes completely 0.8 g of oxygen is formed.
APPEARS IN
संबंधित प्रश्न
Give balanced chemical equations for the following conversions A, B, and C:
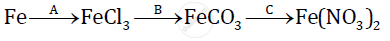
Prove the Following :
2 X V.D. = Molecular mass.
Calculate the relative molecular mass of Ammonium sulphate.
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
Washing soda has the formula Na2CO3.10H2O.What is the mass of anhydrous sodium carbonate left when all the water of crystallization is expelled by heating 57.2 g of washing soda?
The reaction of potassium permanganate (VII) with acidified iron (II) sulphate is given below:
2KMno4 + 10FeSO4 + 8H2O → K2SO4 + 2MnSO4 + 5Fe2(SO4)3 + 8H2O
If 15.8g of potassium permanganate (VII) was used in the reaction, calculate the mass of iron (II) sulphate used in the above reaction.
Calculate the percentage of nitrogen in aluminium nitride. [Al = 27, N = 14]
Calculate the relative molecular mass of:
CH3COONa
Find the weight of 0.2 mole of H2 gas.
The mass of 5.6 litres of a certain gas at S.T.P. is 12 g. What is the relative molecular mass or molar mass of the gas?
When heated, potassium permanganate decomposes according to the following equation :
\[\ce{2KMnO4 -> \underset{\text{solid residue}}{K2MnO4 + MnO2} + O2}\]
(a) Some potassium permanganate was heated in the test tube. After collecting one litre of oxygen at room temperature, it was found that the test tube had undergone a loss in mass of 1.32 g. If one litre of hydrogen under the same conditions of temperature and pressure has a mass of 0.0825 g, calculate the relative molecular mass of oxygen.
(b) Given that the molecular mass of potassium permanganate is 158. What volume of oxygen (measured at room temperature) would be obtained by the complete decomposition of 15.8 g of potassium permanganate? (Molar volume at room temperature is 24 litres)
