Advertisements
Advertisements
प्रश्न
Read the following reaction and answer the questions given below:
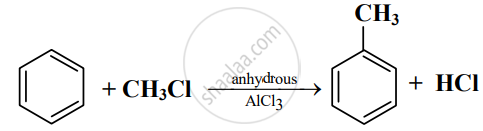
- Write the name of the reaction.
- Identify the electrophile in it.
- How is this electrophile generated?
उत्तर
- The name of the reaction is Friedel-Craft’s alkylation reaction.
- The electrophile in the reaction is +CH3.
- The electrophile +CH3 is generated as follows:
\[\ce{\underset{\text{Methyl chloride}}{CH3 -}Cl + AlCl3 -> \underset{\text{Electrophile}}{^+CH3 +}AlCl^-_4}\]
APPEARS IN
संबंधित प्रश्न
Write the balanced chemical reaction to get benzene from Sodium benzoate.
Identify giving reason whether the following compound is aromatic or not.
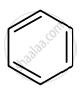
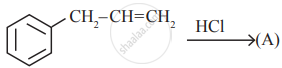 is
is
Which of the following compounds will not undergo Friedal – crafts reaction easily ? (NEET)
Write the chemical equations for combustion of propane.
How does Huckel rule help to decide the aromatic character of a compound?
What happens when Isobutylene is treated with acidified potassium permanganate?
How will you convert ethyl chloride into ethane?
What happens when ethylene is passed through cold dilute alkaline potassium permanganate.
Write the structure of the following alkanes.
2, 3 – Dimethyl – 6 – (2 – methyl propyl) decane

Identify A and B.
Complete the following:
\[\begin{array}{cc}
\ce{CH2 - CH2 ->[Zn/C2H5OH]}\\
|\phantom{.......}|\phantom{..............}\\
\ce{Br}\phantom{.....}\ce{Br}\phantom{..............}
\end{array}\]
Which of the following is NOT a hetero-aromatic compound?
The alkane formed on heating sodium butanoate with sodalime is ______.
Sodium benzoate on decarboxylation gives ____________.
Dow's process is used for the synthesis of an aromatic compound (X). Identify X.
Which of the following reagents can bring about following conversion?
\[\ce{But-1-ene -> Butan-2-ol}\]
Arenes on treatments with chlorine in the presence of ferric chloride as a catalyst undergo what type of reaction?
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr->[Benzoyl][peroxide] H3C - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide]CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}\\
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{.............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}\\
\end{array}\]
- Write IUPAC name of the product.
- State the rule that governs formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH_3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{...............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide]CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{..................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide]CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write IUPAC name of the product.
- State the rule that governs formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{..................................}
\end{array}\]
- Write IUPAC name of the product.
- State the rule that governs formation of this product.
