Advertisements
Advertisements
प्रश्न
Saturated hydrocarbon : Single bond : : Unsaturated hydrocarbon : _______
उत्तर
Saturated hydrocarbon : Single bond : : Unsaturated hydrocarbon : Double and/or triple bond
APPEARS IN
संबंधित प्रश्न
What is a homologous series?
State any four characteristics of a homologous series
Write the name and formula of the 2nd member of homologous series having general formula CnH2n – 2.
Write the molecular formula of first two members of homologous series having functional group – OH
The following vegetables are kept in a basket :
Potato, Tomato, Radish, Brinjal, Carrot, Bottle-gourd
Which two of these vegetables correctly represent the homologous structures?
(A) Carrot and Tomato
(B) Potato and Brinjal
(C) Radish and Carrot
(D) Radish and Bottle-gourd
Write the molecular formula of the 2nd and 3rd member of the homologous series where the first member is ethyne.
What is the difference between two consecutive homologues:
(1) in terms of molecular mass?
(2) in terms of number and kind of atoms per molecule?
Give the molecular formula of one homologue of the following:
C3H6
Write the names and formulae for the first three members of the homologous series for chloroalkanes.
Define a homologous series. Give the name and structural formula of one homologue of the following:
CH3OH
Write the molecular formula of the third member of the homologous series of carbon compounds with general formula CnHO2n+1OH.
Give the abbreviated formula of the third member of the alcohol.
Give the structure of the second member of the alcohol group
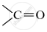 .
.
Define homologous series of organic compounds. List its two characteristics. Write the name and formula of the first member of the series of alkenes.
The general formula of Alkane is _________________
The unsaturated hydrocarbons containing a carbon-carbon double bond are called _______.
Complete the following table for homologous series of Alkenes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Ethene | C2H4 | CH2 = CH2 | 2 | 0 | -102 |
| Propene | C3H6 | CH3–CH = CH2 | 3 | 1 | -48 |
| 1-Butene | C4H8 | CH3–CH2–CH = CH2 | ______ | ______ | -6.5 |
| 1-Pentene | C5H10 | CH3–CH2–CH2–CH = CH2 | ______ | ______ | 30 |
Define Homologous series.
Consider the carbon compounds having following molecular formula:
(i) C3H6 (ii) C3H8 (iii) C4H6 (iv) C6H6 (v) C6H12
- State the number of double covalent bonds present in C3H6.
- Write the formula of first member of the homologous series to which the carbon compound C4H6 belongs.
- Which one of the above compounds forms a ring structure of carbon atoms?
- Identify, which of the above compounds, is a member of alkane series.
