Advertisements
Advertisements
प्रश्न
State the conditions required for the given reaction to take place:
Preparation of ethyne from ethylene dibromide
उत्तर
The condition required for the preparation of ethylene from ethylene dibromide is that the solution should be boiled.
The chemical equation is as follows:
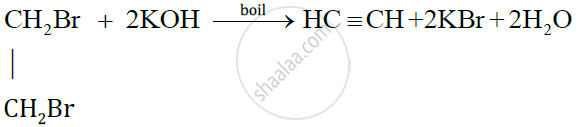
APPEARS IN
संबंधित प्रश्न
Give balanced chemical equations for Calcium carbide to ethyne.
Give a chemical test to distinguish between ethane and ethene.
Give a chemical test to distinguish between ethene (ethylene) and ethyne (acetylene).
Name the products formed and write an equation when ethyne is added to the hydrogen in an
inert solvent ?
Convert ethyne to ethane.
Write an equation for the laboratory preparation of an unsaturated hydrocarbon from calcium carbide.
Name the products formed and write an equation when ethyne is added to the following in an inert solvent:
Bromine
Name the products formed and write an equation when ethyne is added to the following in an inert solvent:
excess of hydrochloric acid
Substitution reactions are characteristic reactions of ______.
Alkynes undergo ______ reactions.
