Advertisements
Advertisements
प्रश्न
The photon emitted during the de-excitation from the first excited level to the ground state of a hydrogen atom is used to irradiate a photocathode in which the stopping potential is 5 V. Calculate the work function of the cathode used.
उत्तर
In the photoelectric effect, stopping potential is directly proportional to the maximum kinetic energy of the emitted electron.
This maximum kinetic energy is further dependent on the frequency of incident light as well as the work function of the cathode. Combining both these facts, we can arrive at the work function of the cathode used.
KE = hν - Φ0
Where, hν = E2 - E1
= `(-13.6)/2^2 - (-13.6)/1^2`
= `3/4 xx 13.6 = 10.2` eV
eV = hν - Φ0
`5V xx e = 10.2 eV - phi_0`
Φ0 = 5.2 eV
APPEARS IN
संबंधित प्रश्न
Light of wavelength 488 nm is produced by an argon laser which is used in the photoelectric effect. When light from this spectral line is incident on the emitter, the stopping (cut-off) potential of photoelectrons is 0.38 V. Find the work function of the material from which the emitter is made.
Define the terms (i) ‘cut-off voltage’ and (ii) ‘threshold frequency’ in relation to the phenomenon of photoelectric effect.
Using Einstein’s photoelectric equation shows how the cut-off voltage and threshold frequency for a given photosensitive material can be determined with the help of a suitable plot/graph.
The electric field at a point associated with a light wave is `E = (100 "Vm"^-1) sin [(3.0 xx 10^15 "s"^-1)t] sin [(6.0 xx 10^15 "s"^-1)t]`.If this light falls on a metal surface with a work function of 2.0 eV, what will be the maximum kinetic energy of the photoelectrons?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
How does one explain the emission of electrons from a photosensitive surface with the help of Einstein’s photoelectric equation?
Use Einstein's photoelectric equation to show how from this graph,
(i) Threshold frequency, and
(ii) Planck's constant can be determined.
Choose the correct answer from given options
Photons of frequency v are incident on the surface of two metals A and B of threshold frequency 3/4 v and 2/3 v, respectively. The ratio of maximum kinetic energy of electrons emitted from A to that from B is
- In the explanation of photo electric effect, we assume one photon of frequency ν collides with an electron and transfers its energy. This leads to the equation for the maximum energy Emax of the emitted electron as Emax = hν – φ0 where φ0 is the work function of the metal. If an electron absorbs 2 photons (each of frequency ν) what will be the maximum energy for the emitted electron?
- Why is this fact (two photon absorption) not taken into consideration in our discussion of the stopping potential?
There are materials which absorb photons of shorter wavelength and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength.
A student performs an experiment on photoelectric effect, using two materials A and B. A plot of Vstop vs ν is given in Figure.
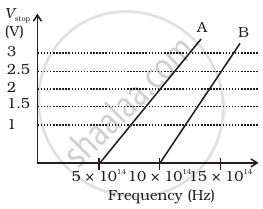
- Which material A or B has a higher work function?
- Given the electric charge of an electron = 1.6 × 10–19 C, find the value of h obtained from the experiment for both A and B.
Comment on whether it is consistent with Einstein’s theory:
A photon of wavelength 663 nm is incident on a metal surface. The work function of the metal is 1.50 eV. The maximum kinetic energy of the emitted photoelectrons is ______.
