Advertisements
Advertisements
प्रश्न
The sketch below illustrates the refin ing of aluminium by Hoope's process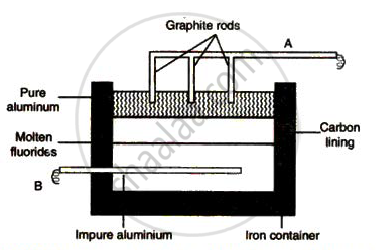
(a) Which of A and B is th e cathode and which one is the anode?
(b) What is the electroly te in the tank?
( c) What material is used for th e cathode?
उत्तर
(a) A is cathode and B is anode.
(b) Molten fluorides of Al, Na and Ba.
(c) Graphite rods.
APPEARS IN
संबंधित प्रश्न
Answer the following questions with respect to the electrolytic process in the extraction of aluminum:
Identify the components of the electrolyte other than pure alumina and the role played by each
Answer the following questions with respect to the electrolytic process in the extraction of aluminum:
Explain why powdered coke is sprinkled over the electrolytic mixture.
Name the constituents of Duralumin.
Name the constituents of Bronze.
For graphite, explain its significance in the extraction of aluminium.
For the substance listed below, explain its role in the extraction of aluminium: Graphite
Aluminium is extracted from its chief ore bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Name a chemicals used for dissolving aluminium oxide. In which state of sub-division is the chemical used?
Write the equation for the reaction where the aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide.
State true or false.
Bauxite is the main ore of aluminium.
The compound that is not a constituent of the electrolytic mixture used in Hall-Heroult's process is ______.
