Advertisements
Advertisements
प्रश्न
The sketch below illustrates the refin ing of aluminium by Hoope's process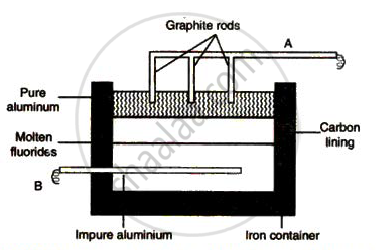
(a) Which of A and B is th e cathode and which one is the anode?
(b) What is the electroly te in the tank?
( c) What material is used for th e cathode?
उत्तर
(a) A is cathode and B is anode.
(b) Molten fluorides of Al, Na and Ba.
(c) Graphite rods.
APPEARS IN
संबंधित प्रश्न
Answer the following questions with respect to the electrolytic process in the extraction of aluminum:
Identify the components of the electrolyte other than pure alumina and the role played by each
Aluminium is a more active metal than iron, but suffers less corrosion. Why?
Name three alloys of steel. Give their compositions and uses.
Name the following:
The chief ore of aluminium.
What is the role of cryolite (NaAlF6) in the electrolytic reduction of alumina in the Hall's process?
Aluminium is a more active metal than iron, but suffers less corrosion. Why?
Explain and give reasons why aluminium vessels should not be cleaned with powders containing alkalis.
Write the constituents of the electrolyte for the extraction of aluminium.
State the relevant reason for the following:
Graphite anodes are continuously replaced during the electrolysis of alumina.
What impurities are present in aluminium ore?
