Advertisements
Advertisements
प्रश्न
What impurities are present in aluminium ore?
उत्तर
Silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2) are the impurities present in aluminium ore.
APPEARS IN
संबंधित प्रश्न
Write the equation for the reaction that occurs at the cathode during the extraction of aluminum by electrolysis.
Name the constituents of Bronze.
Name the following:
The substance added along with aluminium in the Hall-Heroult's process.
Give reason for the following:
Extraction of aluminium was very difficult in the beginning.
In the Hall's process for extraction of aluminium, Give the formula and purpose of fluorspar and cryolite
In the Hall's process for extraction of aluminium, Sate the location of cathode and anode and explain what occurs at each electrode.
Aluminium is a more active metal than iron, but suffers less corrosion. Why?
Explain and give reasons why aluminium vessels should not be cleaned with powders containing alkalis.
Why is food containing iron salts should not be cooked in aluminium utensils?
For the substance listed below, explain its role in the extraction of aluminium: Graphite
In order to obtain one tone of aluminium, the following inputs are required:
4 tones of bauxite, 150 Kg of sodium hydroxide and 600 Kg of graphite.
The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (II) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
(i) Name the process used for purification of bauxite.
(ii) Write the equation to show the action of heat on aluminium hydroxide.
A to F below relate to the source and extraction of either zinc or aluminium.
- Bauxite
- Coke
- Cryolite
- Froth floatation
- Sodium hydroxide solution
- Zinc blende
Fill in the blanks using the most appropriate words from A to F:
(i) The ore from which aluminium is extracted must be treated with ______ so that pure aluminium oxide can be obtained.
(ii) Pure aluminium oxide is dissolved in ______ to make a conducting solution.
Correct the following statement :
Haematite is the chief ore of aluminium.
Describe the role played in the extraction of aluminum:
Cryolite
Explain why :
In the electrolysis of alumina using the Hall Heroult's Process the electrolyte is covered with powdered coke.
In Hoope's process, pure aluminium is collected at the ______ of the electrolytic cell.
Explain with reason:
A neutral gas other than oxygen is formed at the anode during the electrolysis of fused alumina.
Name the alloy used for the following purpose.
Making parts of watches
The following sketch illustrates the process of conversion of Alumina to Aluminium:
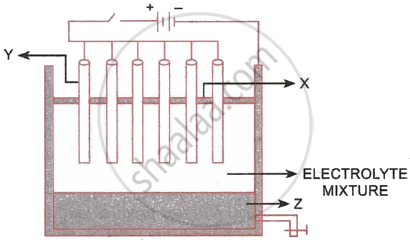
- Name the constituent of the electrolyte mixture which has a divalent metal in it.
- Name the powdered substances ‘X’ sprinkled on the surface of the electrolyte mixture.
- What is the name of the process?
- Write the reactions taking place at the electrodes ‘Y’ (anode) and ‘Z’ (cathode), respectively.
The compound that is not a constituent of the electrolytic mixture used in Hall-Heroult's process is ______.
