Advertisements
Advertisements
प्रश्न
Give reason for the following:
Extraction of aluminium was very difficult in the beginning.
उत्तर
Extraction of aluminium was very difficult in the beginning because it was very expensive.
APPEARS IN
संबंधित प्रश्न
Name the process by which the refining of aluminium is done ?
Both brass and bronze contain copper as major constituents. Name other elements in these alloys.
For bauxite, explain its significance in the extraction of aluminium.
For the substance listed below, explain its role in the extraction of aluminium: Bauxite
The following is an extract from 'Metals in the Service of Man, Alexander and Street/Pelican 1976':
| 'Alumina (aluminium oxide) has a very high melting point of over 2000°C so that it cannot readily be liquefied. However, conversion of alumina to aluminium and oxygen, by electrolysis, can occur when it is dissolved in some other substance.' |
- Which solution is used to react with bauxite as a first step in obtaining pure aluminium oxide?
- The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Write the balanced chemical equation for this reaction.
- Name the element which serves both as the anode and the cathode in the extraction of aluminium.
- Write the balanced chemical equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
- Give the balanced chemical equation for the reaction which occurs at the anode when aluminium is purified by electrolysis.
The sketch below illustrates the refin ing of aluminium by Hoope's process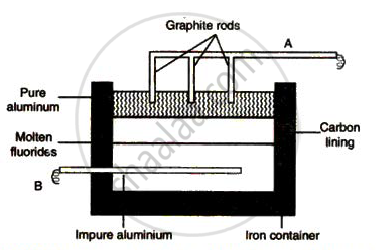
(a) Which of A and B is th e cathode and which one is the anode?
(b) What is the electroly te in the tank?
( c) What material is used for th e cathode?
Name the compound added to pure alumina to lower the fusion temperature during the electrolytic reduction of alumina.
Name the alloy used for the following purpose.
Making parts of watches
Write the balanced chemical equation to show the concentration of ore in Baeyer’s process.
Aluminium hydroxide to alumina
Given below in column A is a schematic diagram of the electrolytic reduction of alumina. Identify the parts labelled as A, B and C with the correct options from the Column B.
| column A | column B | |
 |
1. | Platinum |
| 2. | Anode | |
| 3. | Cathode | |
| 4. | Electrolyte mixture | |
| 5. | Bauxite |
