Advertisements
Advertisements
Question
Give reason for the following:
Extraction of aluminium was very difficult in the beginning.
Solution
Extraction of aluminium was very difficult in the beginning because it was very expensive.
APPEARS IN
RELATED QUESTIONS
Write the equation for the reaction where the aluminum oxide for the electrolytic extraction of aluminum is obtained by heating aluminum hydroxide.
For the substance given below, describe the role played in the extraction of aluminium.
Cryolite
Name the constituents of Bronze.
| Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction. |
Name a chemical used for dissolving aluminium oxide. In which state is the chemical used?
Write balanced equation for the following reaction:
Aluminium powder is warmed with hot and concentrated caustic soda solution.
Aluminium is extracted from its chief ore bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Write three balanced equations for the purification of bauxite by Hall's process.
Aluminium is extracted from its chief ore bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Name a chemicals used for dissolving aluminium oxide. In which state of sub-division is the chemical used?
Explain why :
In the electrolysis of alumina using the Hall Heroult's Process the electrolyte is covered with powdered coke.
Name the alloy used for the following purpose.
Making parts of watches
The following sketch illustrates the process of conversion of Alumina to Aluminium:
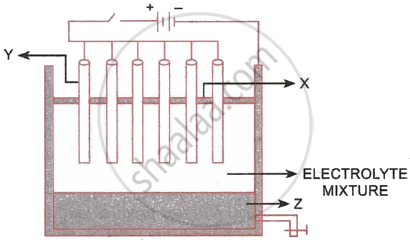
- Name the constituent of the electrolyte mixture which has a divalent metal in it.
- Name the powdered substances ‘X’ sprinkled on the surface of the electrolyte mixture.
- What is the name of the process?
- Write the reactions taking place at the electrodes ‘Y’ (anode) and ‘Z’ (cathode), respectively.
