Advertisements
Advertisements
प्रश्न
What colour do the following indicators turn when added to a base or alkali (such as sodium hydroxide)?
(a) methyl orange
उत्तर १
Methyl orange: Methyl orange changes its colour to yellow when added to a base or an alkali.
उत्तर २
Methyl orange: Methyl orange changes its colour to yellow when added to a base or an alkali.
APPEARS IN
संबंधित प्रश्न
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Which gas is liberated when dilute hydrochloric acid reacts with sodium carbonate?
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise it will be:
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Write the main difference between an acid and a base.
Answer the following question.
Blue litmus solution is added to two test tubes A and B containing dilute HCl and NaOH solution respectively. In which test tube a colour change will be observed? State the colour change and give its reason.
Vinay observed that the stain of curry on a white shirt becomes reddish-brown when soap is scrubbed on it, but it turns yellow again when the shirt is washed with plenty of water. What might be the reason for his observation?
- Soap is acidic in nature
- Soap is basic in nature
- Turmeric is a natural indicator which gives reddish tinge in bases
- Turmeric is a natural indicator which gives reddish tinge in acids
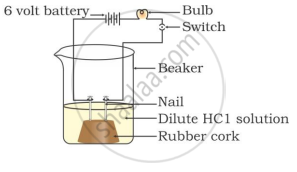
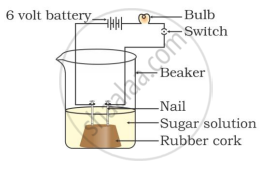
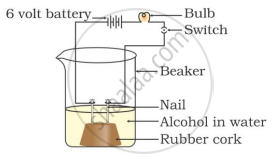
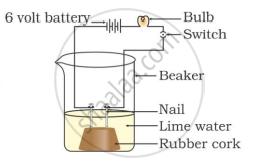
In which of the following setups would the bulb glow?
Which of the following is(are) true when HCl (g) is passed through water?
- It does not ionise in the solution as it is a covalent compound.
- It ionises in the solution
- It gives both hydrogen and hydroxyl ions in the solution
- It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for the removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.