Advertisements
Advertisements
प्रश्न
What do you notice when acetic acid reacts with alkalies?
उत्तर
When acetic acid reacts with alkalies, it forms a salt and water.
\[\ce{\underset{Acetic acid}{CH3COOH} + \underset{alkali}{NaOH} → \underset{Sod. acetate (salt)}{CH3COONa} + \underset{Water}{H2O}}\]
APPEARS IN
संबंधित प्रश्न
Write the name and chemical formula of the simplest organic acid.
Give the name and structural formula of one homologue of HCOOH.
Describe one reaction of a carboxylic acid.
Which of the following molecular formula corresponds to ethylbutanoate ester?
(a) C5H10O2
(b) C6H12O2
(c) C7H14O2
(d) C8H16O2
What do you notice when acetic acid reacts with metals?
Acetic acid smells like:
(1) a banana
(2) vinegar
(3) an orange
(4) a lemon
What type of compound is formed by the reaction between acetic acid and an alcohol?
Explain the following reaction with an example.
Esterification
Observe the diagram given below and answer the questions:
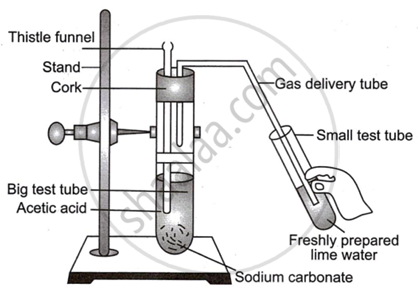
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
