Advertisements
Advertisements
प्रश्न
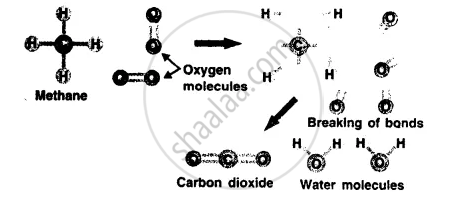
What happens during a chemical reaction ?
उत्तर
A chemical reaction involves breaking of chemical bonds between the atoms or groups of atoms of reacting substances and rearrangement of atoms making new bonds to form new substances.
APPEARS IN
संबंधित प्रश्न
Find the odd man out:
Camphor, Ammonium Chloride, Naphthalene balls, Sugar
What type of chemical reaction take place when a magnesium wire is burnt in air?
Fill in the blank
The chemical reaction between hydrogen and chlorine is a ................ reaction
Explain the terms with examples.
Combination reaction
Calcium oxide reacts vigorously with water to produce slaked lime.
\[\ce{CaO{(s)} + H2O(l) -> Ca(OH)2(aq)}\]
This reaction can be classified as:
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Complete the statement by filling in the blank with the correct word:
Direct combination reaction of phosphorus pentoxide with water gives _______.
Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
Which of the following is not a physical change?
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
Read the text below and answer the questions that follow:
A small amount of hydrochloric acid was taken in a test tube. The test tube was heated. A glass rod was dipped in the ammonia solution and held on the top of the test tube. A white smoke was seen emanating from the tip of the glass rod.
- What must have happened?
- Which colour of gas is formed?
- Write the chemical equation for the reaction.
