Advertisements
Advertisements
प्रश्न
What happens to the position of an element in the periodic table when its nucleus emits β -particle? Give reasons for your answer.
उत्तर
β particles are negatively charged particles with negIigible mass so when a element emits a β particle its mass remain same but atomic number decreases by 1 unit. So after emitting a β particle position of element would shift 1 unit left to the periodic table in same row.
APPEARS IN
संबंधित प्रश्न
What kind of change takes place in a nucleus when a β - particle is emitted? Express it by an equation. State whether
- atomic number and
- mass number are conserved in a radioactive β - decay?
A nucleus is \[\ce {^24_11 Na} \] β-radioactive.
What general name is given to the product nucleus with respect to \[\ce{^24_11 Na}\]?
In β-emission from a radioactive substance, an electron is ejected. This electron comes from ______.
One roentgen is equal to _______ disintegrations per second.
Define critical mass.
A radioactive nucleus containing 128 nucleons emits a β-particle. After β- emission the number of nucleons present in the nucleus will be ______.
|
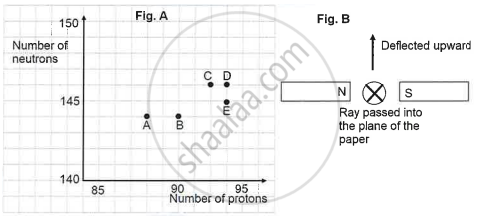
The graph (fig A) illustrates the correlation between the number of protons (x-axis) and the number of neutrons (y-axis) for elements A, B, C, D, and E in the periodic table. These elements are denoted by the letters rather than their conventional symbols. When the element C, depicted in the graph, undergoes radioactive decay, it releases radioactive rays. When these rays are directed into the plane of the paper in the presence of a magnetic field, as indicated in the fig B, they experience deflection, causing them to move upwards.
|
Name the radioactive radiations emitted by the element C.
|
The graph (fig A) illustrates the correlation between the number of protons (x-axis) and the number of neutrons (y-axis) for elements A, B, C, D, and E in the periodic table. These elements are denoted by the letters rather than their conventional symbols. When the element C, depicted in the graph, undergoes radioactive decay, it releases radioactive rays. When these rays are directed into the plane of the paper in the presence of a magnetic field, as indicated in the fig B, they experience deflection, causing them to move upwards.
|
Identify the daughter element from the graph.
What changes occur in the nucleus of a radioactive element when it emits a beta particle. Give one example, in support of your answer.
A nucleus \[\ce{^24_11Na}\] is β-radioactive.
Write the equation representing β-decay.