Advertisements
Advertisements
प्रश्न
|
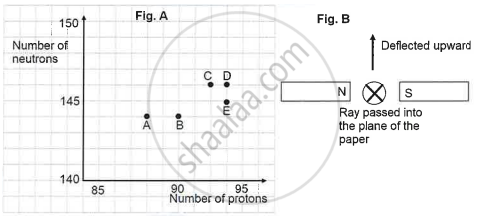
The graph (fig A) illustrates the correlation between the number of protons (x-axis) and the number of neutrons (y-axis) for elements A, B, C, D, and E in the periodic table. These elements are denoted by the letters rather than their conventional symbols. When the element C, depicted in the graph, undergoes radioactive decay, it releases radioactive rays. When these rays are directed into the plane of the paper in the presence of a magnetic field, as indicated in the fig B, they experience deflection, causing them to move upwards.
|
Identify the daughter element from the graph.
उत्तर
Explanation:
Different kinds of radiation can be released by elements during radioactive decay. The kind of radiation released is indicated by the way the rays in a magnetic field are deflected. Positively charged alpha particles would be deflected below, negatively charged beta particles would be deflected upward, and neutral gamma rays would not be deflected. The upward deflection of the beams suggests that element C emits beta radiation. The element with one fewer proton than element C is the daughter element, which may be found by examining the graph. The law of conservation of charge, which stipulates that the total charge before and after the decay must be constant, is used to determine the kind of radiation.
Step 1:
Based on the rays' deflection, determine the kind of radiation that element C emits. The rays are beta particles because they travel upward when a magnetic field is present.
Step 2:
Look at the graph to determine the daughter element. If element C has a certain number of protons, the daughter element will have one less proton.
Step 3:
State the law used to identify the radiation type, which is the law of conservation of charge.
a: Beta radiation b: Element D (assuming it has one less proton than C) c: Law of conservation of charge
APPEARS IN
संबंधित प्रश्न
What happens to the position of an element in the periodic table when its nucleus emits β -particle? Give reasons for your answer.
What kind of change takes place in a nucleus when a β - particle is emitted? Express it by an equation. State whether
- atomic number and
- mass number are conserved in a radioactive β - decay?
A nucleus is \[\ce {^24_11 Na} \] β-radioactive.
What general name is given to the product nucleus with respect to \[\ce{^24_11 Na}\]?
In β-emission from a radioactive substance, an electron is ejected. This electron comes from ______.
One roentgen is equal to _______ disintegrations per second.
Define critical mass.
A radioactive nucleus containing 128 nucleons emits a β-particle. After β- emission the number of nucleons present in the nucleus will be ______.
|
The graph (fig A) illustrates the correlation between the number of protons (x-axis) and the number of neutrons (y-axis) for elements A, B, C, D, and E in the periodic table. These elements are denoted by the letters rather than their conventional symbols. When the element C, depicted in the graph, undergoes radioactive decay, it releases radioactive rays. When these rays are directed into the plane of the paper in the presence of a magnetic field, as indicated in the fig B, they experience deflection, causing them to move upwards.
|
Name the radioactive radiations emitted by the element C.
When happens to the (i) atomic number, (ii) mass number of the nucleus of an element when a β-particle is emitted ?
A nucleus \[\ce{^24_11Na}\] is β-radioactive.
Write the equation representing β-decay.