Advertisements
Advertisements
प्रश्न
What is meant by the following term? Give an example of the reaction in the following case.
2, 4-DNP-derivative
उत्तर
When an aldehyde or ketone reacts with 2, 4-dinitrophenylhydrazine in a weak acidic medium, 2, 4-dinitrophenylhydrazone (2, 4-DNP derivative) is produced.
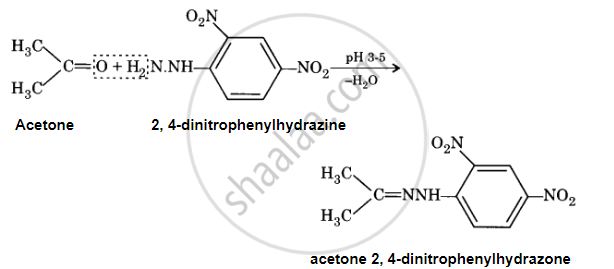
APPEARS IN
संबंधित प्रश्न
Predict the product of the following reaction:
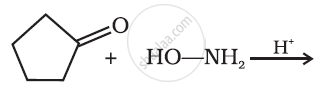
Predict the product of the following reaction:
\[\begin{array}{cc}
\phantom{..............}\ce{O}\\
\phantom{..............}||\\
\ce{R - CH = CH - CHO + NH2 - C - NH - NH2 ->[H+]}\end{array}\]
Complete the synthesis by giving missing starting material, reagent or product.
\[\ce{C6H5CHO ->[H2NCONHNH2]}\]
Write balanced chemical equations for action of ammonia on - acetaldehyde
Give a simple chemical test to distinguish between 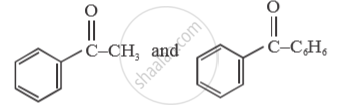
Write the main product formed when propanal reacts with the following reagents:
2 moles of 3 CH OH in presence of dry HCl
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Which one of the following gives only one monochloro derivative?
What is the action of sodium hypoiodite on acetone?
What happens when ethanal is treated with excess ethanol and acid?
In the following reaction
\[\ce{Carbonyl compound + MeOH <=>[HCl] acetal}\]
Rate of the reaction is the highest for ______.
The following questions are case-based questions. Read the passage carefully and answer the questions that follow:
| The carbon-oxygen double bond is polarised in aldehydes and ketones due to higher electronegativity of oxygen relative to carbon. Therefore, they undergo nucleophilic addition reactions with a number of nucleophiles such as HCN, NaHSO3, alcohols, ammonia derivatives and Grignard reagents. Aldehydes are easily oxidised by mild oxidising agents as compared to ketones. The carbonyl group of carboxylic acid does not give reactions of aldehydes and ketones. Carboxylic acids are considerably more acidic than alcohols and most of simple phenols. |
Answer the following:
(a) Write the name of the product when an aldehyde reacts with excess alcohol in the presence of dry HCl. (1)
(b) Why carboxylic acid is a stronger acid than phenol? (1)
(c) (i) Arrange the following compounds in increasing order of their reactivity towards CH3MgBr: (1)
CH3CHO, \[\begin{array}{cc}
\ce{(CH3)3C-C-CH3}\\
\phantom{....}||\\
\phantom{....}\ce{O}
\end{array}\], \[\begin{array}{cc}
\ce{CH3-C-CH3}\\
||\\
\ce{O}
\end{array}\]
(ii) Write a chemical test to distinguish between propanal and propanone. (1)
OR
(c) Write the main product in the following: (2)
| (i) | 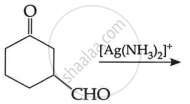 |
| (ii) | 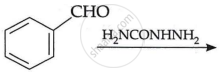 |
Draw structures of the given derivatives.
The ethylene ketal of hexan-3-one
The product of the following reaction is
\[\begin{array}{cc}
\ce{O}\phantom{.........}\\
||\phantom{.........}\\
\ce{C2H5 - C - CH3 ->[H2/Ni][\Delta] \phantom{..}?}\end{array}\]
Draw the structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structure of the following derivative:
Acetaldehydedimethylacetal
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Draw structure of the following derivative.
The ethylene ketal of hexan-3-one
Give an example of the reaction in the following case.
Imine
