Advertisements
Advertisements
प्रश्न
When D-glucose reacts with HI, it forms ______.
विकल्प
Gluconic acid
n-hexane
Saccharic acid
Iodohexane
उत्तर
When D-glucose reacts with HI, it forms n-hexane.
Explanation:
On prolonged heating with HI, it forms n-hexane, suggesting that all the six carbon atoms are linked in a straight chain.
\[\begin{array}{cc}
\ce{CHO}\phantom{.................................................}\\
|\phantom{.....................................................}\\
\ce{(CHOH)4 ->[HI,\Delta] \underset{{(n-Hexane)}}{CH3 - CH2 - CH2 - CH2 - CH2 - CH3}}\\
|\phantom{.....................................................}\\
\ce{CH2OH}\phantom{...............................................}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
Write the reactions involved when D-glucose is treated with the following reagent:
H2N-OH
The following compound can be called as:
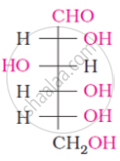
Choose the appropriate answer(s) for the below representation from the options given
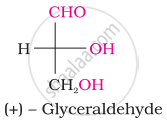
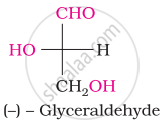
What is the most abundant organic compound on earth?
Acetylation of glucose yields ____________.
When glucose reacts with bromine water, the main product is ____________.
Glucose does not react with ____________.
Which one of the following reactions is not explained by the open chain Structure of glucose?
How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
Account for the following:
There are 5 OH groups in glucose
