Advertisements
Advertisements
प्रश्न
Which of the following compounds will not exist as resonance hybrid. Give reason for your answer:
विकल्प
CH3OH
R – CONH2
CH3CH = CHCH2NH2
उत्तर
CH3OH
Explanation:
(i) CH3OH does not contain -electrons, hence, it cannot exist as resonance hybrid.
(ii) Due to the presence of -electrons in C = O bond and lone pair of electrons on N, amide can be represented by the following resonating structures.
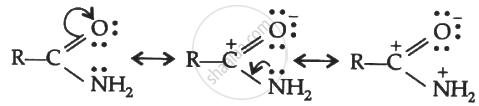
(iii) CH3CH = CHCH2NH2: Since lone pair of electrons on nitrogen atom is not conjugated with the -electrons, therefore, resonance is not possible.
APPEARS IN
संबंधित प्रश्न
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5OH
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
Draw the possible resonance structures for \[\ce{CH3 - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - \overset{+}{C}H2}\] and predict which of the structures is more stable. Give reason for your answer.
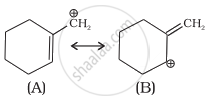
Which of the following ions is more stable? Use resonance to explain your answer.
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.

Draw the resonance structure of the following compounds;
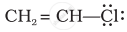
Draw the resonance structure of the following compounds;
CH2 = CH – CH = CH2
Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
\[\ce{\underset{(A)}{CH3COOH}}\] and \[\ce{\underset{(B)}{CH3CO\overset{Θ}{O}}}\]
