Advertisements
Advertisements
प्रश्न
Which of the following compounds will not exist as resonance hybrid. Give reason for your answer:
पर्याय
CH3OH
R – CONH2
CH3CH = CHCH2NH2
उत्तर
CH3OH
Explanation:
(i) CH3OH does not contain -electrons, hence, it cannot exist as resonance hybrid.
(ii) Due to the presence of -electrons in C = O bond and lone pair of electrons on N, amide can be represented by the following resonating structures.
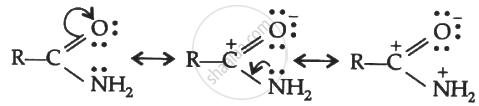
(iii) CH3CH = CHCH2NH2: Since lone pair of electrons on nitrogen atom is not conjugated with the -electrons, therefore, resonance is not possible.
APPEARS IN
संबंधित प्रश्न
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5NO2
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5 – CHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{C6H5 - \overset{+}{C}H2}\]
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
In which of the following representations given below spatial arrangement of group/ atom different from that given in structure ‘A’?
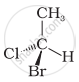 |
| (A) |
| (i) | 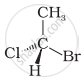 |
| (ii) | 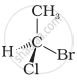 |
| (iii) | 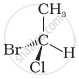 |
| (iv) | 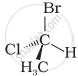 |
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.

Draw the resonance structure of the following compounds;
\[\begin{array}{cc}
\ce{CH2 = CH - C = O}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\]
Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
\[\ce{\underset{(A)}{CH3COOH}}\] and \[\ce{\underset{(B)}{CH3CO\overset{Θ}{O}}}\]
Assertion (A): Energy of resonance hybrid is equal to the average of energies of all canonical forms.
Reason (R): Resonance hybrid cannot be presented by a single structure.
